Abstract
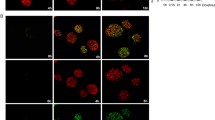
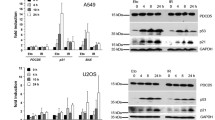
DNA damage in eukaryotic cells induces signaling pathways mediated by the ATM, p53 and ERK proteins, but the interactions between these pathways are not completely known. To address this issue, we performed a time course analysis in human embryonic fibroblast cells treated with DNA-damaging agents. DNA damage induced the phosphorylation of p53 at Ser 15 (p-p53) and the phosphorylation of ERK (p-ERK). Inhibition of p53 by a dominant negative mutant or in p53−/− fibroblast cells abolished ERK phosphorylation. ERK inhibitor prevented p53 phosphorylation, indicating that phosphorylations of p53 and p-ERK are interdependent each other. A time course analysis showed that ATM interacted with p-p53 and p-ERK in early time (0.5 h) and interaction between ATM-bound p-p53 and p-ERK or ATM-bound p-ERK and p-p53 occurred in late time (3 h) of DNA damage. These results indicate that ATM mediates interdependent activation of p53 and ERK through formation of a ternary complex between p-p53 and p-ERK in response to DNA damage to cause growth arrest.





Similar content being viewed by others
References
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506
Cheng Q, Chen J (2010) Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle 9:472–478
Vousden KH (2002) Activation of the p53 tumor suppressor protein. Biochim Biophys Acta 1602:47–59
Hollstein M, Hainaut P (2010) Massively regulated genes: the example of TP53. J Pathol 220:164–173
Appella E, Anderson CW (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268:2764–2772
Persons DL, Yazlovitskaya EM, Pelling JC (2000) Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem 275:35778–35785
Shieh SY, Ikeda M, Taya Y, Prives C (1997) DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325–334
Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387:299–303
Ramos JW (2008) The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol 40:2707–2719
Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ (2002) ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem 277:12710–12717
Mebratu Y, Tesfaigzi Y (2009) How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle 8:1168–1175
She QB, Chen N, Dong Z (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem 275:20444–20449
Kim HS, Yeo EJ, Park SH, Park JI, Park SC, Shin JY, Kim MJ, Oh SJ, Won MH, Kang TC, Park JB, Kim J, Kim JI, Lee HY, Lee JY (2005) p21WAF/CIP1/SDI1 is upregulated due to increased mRNA stability during hydroxyurea-induced senescence of human fibroblasts. Mech Ageing Dev 126:1255–1261
Montecucco A, Biamonti G (2007) Cellular response to etoposide treatment. Cancer Lett 252:9–18
Cohen SM, Lippard SJ (2001) Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol 67:93–130
Pommier Y (2009) DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev 109:2894–2902
Lee SW, Fang L, Igarashi M, Ouchi T, Lu KP, Aaronson SA (2000) Sustained activation of Ras/Raf/mitogen-activated protein kinase cascade by the tumor suppressor p53. Proc Natl Acad Sci U S A 97:8302–8305
Lin T, Mak NK, Yang MS (2008) MAPK regulate p53-dependent cell death induced by benzo[a]pyrene: involvement of p53 phosphorylation and acetylation. Toxicology 247:145–153
Alkhalaf M, Jaffal S (2006) Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med 41:318–325
Ward I, Chen J (2004) Early events in the DNA damage response. Curr Top Dev Biol 63:1–35
Riches LC, Lynch AM, Gooderham NJ (2008) Early events in the mammalian response to DNA double-strand breaks. Mutagenesis 23:331–339
Acknowledgments
This work was supported partly by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0030750). This work was also supported partly by a grant of “Human Resource Development Center for Economic Region Leading Industry” directed by the Ministry of Education, Science & Technology (MEST) and the National Research Foundation of Korea (NRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jee-In Heo, Soo-Jin Oh contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heo, JI., Oh, SJ., Kho, YJ. et al. ATM mediates interdependent activation of p53 and ERK through formation of a ternary complex with p-p53 and p-ERK in response to DNA damage. Mol Biol Rep 39, 8007–8014 (2012). https://doi.org/10.1007/s11033-012-1647-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1647-3