Abstract
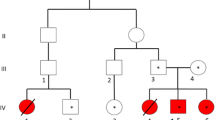
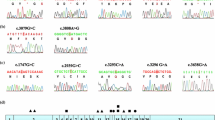
Canavan disease (OMIM 271900) is an autosomal recessive lethal neurodegenerative disorder characterized by spongy degeneration of the brain. A highly consanguineous Pakistani family with Canavan disease was enrolled on the basis of diagnosis. All the affected individuals have mental retardation, megalocephaly and degradation of motor skills, poor head control, partial vision loss, weakness of the muscles and raised urinary concentration of N-acetyl aspartic acid in the urine. Blood samples were collected from affected as well as normal siblings and processed for DNA purification. Linkage analysis was performed by typing three short tandem repeat markers D17S1583 (7.19 cM), D17S1828 (10.02 cM) and D17S919 (14.69 cM) for an already-reported gene/locus ASPA at chromosome 17p13.2 causing Canavan disease. During linkage analysis, all the affected individuals were homozygous for short tandem repeat markers while the normal siblings were heterozygous showing co-segregation of the disease. Gene ASPA (NM_000049) was undertaken to sequence for mutation analysis. As a result of sequence analysis, we found missense substitution 740A→G (p.G274R) in exon 6 of gene ASPA. To our knowledge, this is the first report about Canavan disease on a Pakistani family.



Similar content being viewed by others
References
Matalon R, Michals K, Kaul R (1995) Canavan disease: from spongy degeneration to molecular analysis. J Pediatr 127:511–517
Matalon R, Michals K, Sebasta D, Deanching M, Gashkoff P, Casanova J (1988) Aspartoacylase deficiency in N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet 29:463–471
Birnbaum SM (1955) Aminoacylases I and II from hog kidney. Methods Enzymol 2:115–119
Goldstein FB (1959) Biosynthesis of N-acetyl-l-aspartic acid. J Biol Chem 234:2702–2706
Goldstein FB (1969) The enzymatic synthesis of N-acetyl-l-aspartic acid by subcellular preparations of rat brain. J Biol Chem 244:4257–4260
Shaag A, Anikster Y, Christensen E et al (1995) The molecular basis of canavan (aspartoacylase deficiency) disease in European non-Jewish patients. Am J Hum Genet 57:572–580
Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21:577–581
Zeng BJ, Pastores GM, Leone P, Raghavan S, Wang ZH, Ribeiro LA, Torres P, Ong E, Kolodny EH (2006) Mutation analysis of the aspartoacylase gene in non-Jewish patients with Canavan disease. Adv Exp Med Biol 576:165–173
Elpeleg ON, Anikster Y, Barash V, Branski D, Shaag A (1994) The frequency of the C854 mutation in the aspartoacylase gene in Ashkenazi Jews in Israel. Am J Hum Genet 55:287–288
Feigenbaum A, Moore R, Clarke J, Hewson S, Chitayat D, Ray PN, Stockley TL (2004) Canavan disease: carrier-frequency determination in the Ashkenazi Jewish population and development of a novel molecular diagnostic assay. Am J Med Genet 124:142–147
Kaul R, Gao GP, Aloya M, Balamurugan K, Petrosky A, Michals K, Matalon R (1994) Canavan disease: mutations among Jewish and non-Jewish patients. Am J Hum Genet 55:34–41
Kaul R, Gao GP, Matalon R, Aloya M, Su Q, Jin M, Johnson AB, Schutgens RB, Clarke JT (1996) Identification and expression of eight novel mutations among non-Jewish patients with Canavan disease. Am J Hum Genet 59:95–102
Kronn D, Oddoux C, Phillips J, Ostrer H (1995) Prevalence of Canavan disease heterozygotes in the New York metropolitan Ashkenazi Jewish population. Am J Hum Genet 57:1250–1252
Elpeleg ON, Shaag A (1999) The spectrum of mutations of the aspartoacylase gene in Canavan disease in non-Jewish patients. J Inherit Metab Dis 22:531–534
Janson CG, Kolodny EH, Zeng BJ et al (2006) Mild-onset presentation of Canavan’s disease associated with novel G212A point mutation in aspartoacylase gene. Ann Neurol 59:428–431
Sistermans EA, de Coo RF, van Beerendonk HM, Poll-The BT, Kleijer WJ, van Oost BA (2000) Mutation detection in the aspartoacylase gene in 17 patients with Canavan disease: four new mutations in the non-Jewish population. Eur J Hum Genet 8:557–560
Yalcinkaya C, Benbir G, Salomons GS, Karaarslan E, Rolland MO, Jakobs C, van der Knaap MS (2005) Atypical MRI findings in Canavan disease: a patient with a mild course. Neuropediatrics 36:336–339
Zeng BJ, Wang ZH, Ribeiro LA et al (2002) Identification and characterization of novel mutations of the aspartoacylase gene in non-Jewish patients with Canavan disease. J Inherit Metab Dis 25:557–570
Zeng BJ, Pastores GM, Leone P, Raghavan S, Wang ZH, Ribeiro LA, Torres P, Ong E, Kolodny EH (2006) Mutation analysis of the aspartoacylase gene in non-Jewish patients with Canavan disease. Adv Exp Med Biol 576:165–173
Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A (1989) A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res 17:8390
Lindner TH, Hoffmann K (2005) easyLINKAGE: a PERL script for easy and automated two-/multi-point linkage analyses. Bioinformatics 21:405–407
Tacke U, Olbrich H, Sass JO, Fekete A, Horvath J, Ziyeh S, Kleijer WJ, Rolland MO, Fisher S, Payne S, Vargiami E, Zafeiriou DI, Omran H (2005) Possible genotype-phenotype correlations in children with mild clinical course of Canavan disease. Neuropediatrics 36:252–255
Hershfield JR, Pattabiraman N, Madhavarao CN, Namboodiri MA (2007) Mutational analysis of aspartoacylase: implications for Canavan disease. Brain Res 1148:1–14
Cygler M, Schrag JD, Sussman JL, Harel M, Silman I, Gentry MK, Doctor BP (1993) Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins. Protein Sci 2:366–382
Kaul R, Gao GP, Balamurugan K, Matalon R (1993) Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet 5:118–123
Matalon R, Michals-Matalon K (1999) Recent advances in Canavan disease. Adv Pediatr 46:493–506
Matalon R, Michals K, Kaul R (1995) Canavan disease: from spongy degeneration to molecular analysis. J Pediatr 127:511–517
Matalon R, Michals K, Sebesta D, Deanching M, Gashkoff P, Casanova J (1988) Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet 29:463–471
Acknowledgments
We wish to thank all the participants during this research. This study was supported by Higher Education Commission (HEC) of Pakistan through research grant (20-1138/R&d/08/2383) under program of NRPU.
Electronic databases
Online Mendelian inheritance in Man (http://www.ncbi.nlm.nih.gov/sites/entrez?db=omim). Marshfield genetic map (http://research.marshfieldclinic.org/). Primer3 web site (http://frodo.wi.mit.edu.cgi-bin/primer3_www.cgi).
Author information
Authors and Affiliations
Corresponding author
Additional information
Rashida Hussain and Shakeela Daud have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Hussain, R., Daud, S., Kakar, N. et al. A missense mutation (p.G274R) in gene ASPA causes Canavan disease in a Pakistani family. Mol Biol Rep 39, 6197–6201 (2012). https://doi.org/10.1007/s11033-011-1438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1438-2