Abstract
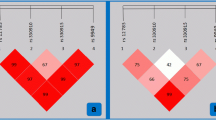
The purinergic 1 receptor (P2RY1) has been implicated in development of heart disease and in individual pharmacodynamic response to anticoagulant therapies. However, the association of polymorphisms in the P2RY1 gene with myocardial infarction (MI), and its associated conditions, has yet to be reported in the literature. We evaluated seven known SNPs in P2RY1 for association with MI in a Latvian population. Seven independent parameters that are related to MI [body mass index (BMI), type 2 diabetes (T2D), angina pectoris, hypertension, hyperlipidemia, atrial fibrillation and heart failure] were investigated. No significant association with MI was observed for any of the polymorphisms. Those SNPs for which the P value was close to significance were located in coding or promoter regions. Intriguingly, carriers of the minor allele in the P2RY1 gene locus showed a tendency towards higher onset age for MI, suggesting a possible protective effect of these SNPs against MI or their contribution in progression as opposed to onset. Finally, a linkage disequilibrium (LD) plot was generated for these polymorphisms in the Latvian population. The results of this study suggest that the role of P2RY1 in individuals from Latvian population is likely to be principally involved in platelet aggregation and thromboembolic diseases, and not as a significant contributing factor to the global metabolic syndrome.



Similar content being viewed by others
References
Ayyanathan K, Webbs TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP (1996) Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun 218(3):783–788. doi:10.1016/j.tips.2006.01.005
Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems JM (1996) Cloning and tissue distribution of the human P2Y1 receptor. Biochem Biophys Res Commun 221(3):588–593
Leon C, Vial C, Cazenave JP, Gachet C (1996) Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoceptor. Gene 171(2):295–297. doi:10.1016/0378-1119(96)00027-3
Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR (2001) Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521(1–3):107–119
Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C (1999) Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest 104(12):1731–1737. doi:10.1172/JCI8399
Malin SA, Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain 6:21. doi:10.1186/1744-8069-6-21
Reiser G (1995) Ca(2+)- and nitric oxide-dependent stimulation of cyclic GMP synthesis in neuronal cell line induced by P2-purinergic/pyrimidinergic receptor. J Neurochem 64(1):61–68
Ryten M, Yang SY, Dunn PM, Goldspink G, Burnstock G (2004) Purinoceptor expression in regenerating skeletal muscle in the mdx mouse model of muscular dystrophy and in satellite cell cultures. FASEB J 18(12):1404–1406. doi:10.1096/fj.03-1175fje03-1175fje
Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409(6817):202–207. doi:10.1038/35051599
Savage B, Saldivar E, Ruggeri ZM (1996) Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84(2):289–297. doi:10.1016/S0092-8674(00)80983-6
Mills DC, Robb IA, Roberts GC (1968) The release of nucleotides, 5-hydroxytryptamine and enzymes from human blood platelets during aggregation. J Physiol 195(3):715–729
Park HS, Hourani SM (1999) Differential effects of adenine nucleotide analogues on shape change and aggregation induced by adnosine 5-diphosphate (ADP) in human platelets. Br J Pharmacol 127(6):1359–1366. doi:10.1038/sj.bjp.0702690
Gachet C (2006) Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol 46:277–300. doi:10.1146/annurev.pharmtox.46.120604.141207
Halbrugge M, Friedrich C, Eigenthaler M, Schanzenbacher P, Walter U (1990) Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem 265(6):3088–3093
Gurbel PA, O’Connor CM, Cummings CC, Serebruany VL (1999) Clopidogrel: the future choice for preventing platelet activation during coronary stenting? Pharmacol Res 40(2):107–111. doi:10.1006/phrs.1999.0478S1043-6618(99)90478-4
Jauhar R, Bergman G, Savino S, Deutsch E, Shaknovich A, Parikh M, Sanborn TA (1999) Effectiveness of aspirin and clopidogrel combination therapy in coronary stenting. Am J Cardiol 84(6):726–728, A8
Azarpira N, Namazi S, Khalili A, Tabesh M (2010) The investigation of allele and genotype frequencies of CYP3A5 (1*/3*) and P2Y12 (T744C) in Iran. Mol Biol Rep. doi:10.1007/s11033-010-0628-7
Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P (2003) Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108(8):989–995. doi:10.1161/01.CIR.0000085073.69189.8801.CIR.0000085073.69189.88
Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL (2003) P2Y12 H2 haplotype is associated with peripheral arterial disease: a case–control study. Circulation 108(24):2971–2973. doi:10.1161/01.CIR.0000106904.80795.35
Rudez G, Pons D, Leebeek F, Monraats P, Schrevel M, Zwinderman A, de Winter R, Tio R, Doevendans P, Jukema W, de Maat M (2008) Platelet receptor P2RY12 haplotypes predict restenosis after percutaneous coronary interventions. Hum Mutat 29(3):375–380. doi:10.1002/humu.20641
Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT (2009) Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol 133(3):341–345. doi:10.1016/j.ijcard.2007.12.118
Gachet C, Leon C, Hechler B (2006) The platelet P2 receptors in arterial thrombosis. Blood Cells Mol Dis 36(2):223–227. doi:10.1016/j.bcmd.2005.12.024
Rozalski M, Nocun M, Watala C (2005) Adenosine diphosphate receptors on blood platelets: potential new targets for antiplatelet therapy. Acta Biochim Pol 52(2):411–415
Hetherington SL, Singh RK, Lodwick D, Thompson JR, Goodall AH, Samani NJ (2005) Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler Thromb Vasc Biol 25(1):252–257
Fontana P, Remones V, Reny JL, Aiach M, Gaussem P (2005) P2Y1 gene polymorphism and ADP-induced platelet response. J Thromb Haemost 3(10):2349–2350. doi:10.1111/j.1538-7836.2005.01483.x
Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS (2007) Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res 119(3):355–360. doi:10.1016/j.thromres.2006.02.006
Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N (2006) P2Y1 gene A1622G dimorphism is not associated with adenosine diphosphate-induced platelet activation and aggregation after administration of a single high dose of clopidogrel. J Thromb Haemost 4(4):912–914
Li Q, Chen BL, Ozdemir V, Ji W, Mao YM, Wang LC, Lei HP, Fan L, Zhang W, Liu J, Zhou HH (2007) Frequency of genetic polymorphisms of COX1, GPIIIa and P2Y1 in a Chinese population and association with attenuated response to aspirin. Pharmacogenomics 8(6):577–586. doi:10.2217/14622416.8.6.577
Jefferson BK, Foster JH, McCarthy JJ, Ginsburg G, Parker A, Kottke-Marchant K, Topol EJ (2005) Aspirin resistance and a single gene. Am J Cardiol 95(6):805–808
Kunicki TJ, Williams SA, Nugent DJ, Harrison P, Segal HC, Syed A, Rothwell PM (2009) Lack of association between aspirin responsiveness and seven candidate gene haplotypes in patients with symptomatic vascular disease. Thromb Haemost 101(1):123–133
Kunicki TJ, Williams SA, Salomon DR, Harrison P, Crisler P, Nakagawa P, Mondala TS, Head SR, Nugent DJ (2009) Genetics of platelet reactivity in normal, healthy individuals. J Thromb Haemost 7(12):2116–2122
Storey RF, Melissa Thornton S, Lawrance R, Husted S, Wickens M, Emanuelsson H, Cannon CP, Heptinstall S, Armstrong M (2009) Ticagrelor yields consistent dose-dependent inhibition of ADP-induced platelet aggregation in patients with atherosclerotic disease regardless of genotypic variations in P2RY12, P2RY1, and ITGB3. Platelets 20(5):341–348
Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, Scheet P, Gwinn M, Williamson RE, Zou GY, Hutchings K, Johnson CY, Tait V, Wiens M, Golding J, van Duijn C, McLaughlin J, Paterson A, Wells G, Fortier I, Freedman M, Zecevic M, King R, Infante-Rivard C, Stewart AF, Birkett N (2009) Strengthening the reporting of genetic association studies (STREGA): an extension of the strengthening the reporting of observational studies in epidemiology (STROBE) statement. J Clin Epidemiol 62(6):597–608
Barrett JC (2009) Haploview: visualization and analysis of SNP genotype data. Cold Spring Harb Protoc 2009(10). doi:10.1101/pdb.ip71
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2):263–265. doi:10.1093/bioinformatics/bth457bth457
de Bakker PI (2009) Selection and evaluation of Tag-SNPs using Tagger and HapMap. Cold Spring Harb Protoc 2009(6). doi:10.1101/pdb.ip67
Kirsten H, Dienst S, Emmrich F, Ahnert P (2006) CalcDalton: a tool for multiplex genotyping primer design for single-base extension reactions using cleavable primers. Biotechniques 40(2):158
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575. doi:10.1086/519795
Gauderman WJ, Morrison JM (2006) QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. http://hydra.usc.edu/gxe
Ecke D, Hanck T, Tulapurkar ME, Schafer R, Kassack M, Stricker R, Reiser G (2008) Hetero-oligomerization of the P2Y11 receptor with the P2Y1 receptor controls the internalization and ligand selectivity of the P2Y11 receptor. Biochem J 409(1):107–116
Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME (2007) Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 293(3):H1609–H1616
Ahmed W, Malik M, Saeed I, Khan AA, Sadeque A, Kaleem U, Ahmed N, Ajmal M, Azam M, Qamar R (2010) Role of tissue plasminogen activator and plasminogen activator inhibitor polymorphism in myocardial infarction. Mol Biol Rep 38(4):2541–2548. doi:10.1007/s11033-010-0392-8
Bronic A, Ferencak G, Zadro R, Stavljenic-Rukavina A, Bernat R (2009) Impact of FXIII-A Val34Leu polymorphism on coronary artery disease in Croatian patients. Mol Biol Rep 36(1):1–5. doi:10.1007/s11033-007-9144-9
Jin B, Li Y, Ge-Shang QZ, Ni HC, Shi HM, Shen W (2010) Varied association of prothrombin G20210A polymorphism with coronary artery disease susceptibility in different ethnic groups: evidence from 15,041 cases and 21,507 controls. Mol Biol Rep 38(4):2371–2376. doi:10.1007/s11033-010-0370-1
Acknowledgments
This study was supported by grants from the Latvian Council of Science (LZPSP10.0010.10.04) and Latvian Research Program (4VPP-2010-2/2.1). In addition, partial funding support was provided by the European Science Foundation (1DP/1.1.1.2.0/09/APIA/VIAA/150 to V.I., K.M., and R.P.) and the Swedish Research Council (H.B.S.). We acknowledge the Genome Database of Latvian Population, Latvian Biomedical Research and Study Centre for providing data and DNA samples.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ignatovica, V., Latkovskis, G., Peculis, R. et al. Single nucleotide polymorphisms of the purinergic 1 receptor are not associated with myocardial infarction in a Latvian population. Mol Biol Rep 39, 1917–1925 (2012). https://doi.org/10.1007/s11033-011-0938-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0938-4