Abstract
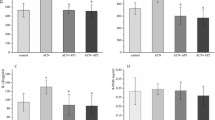
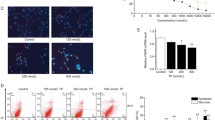
The aim of this study is to explore the mechanism by which acrolein (ACR), a metabolite of cyclophosphamide (CP), induces immature Sertoli cell cytoskeletal changes. Sertoli cells obtained from rats were cultivated and treated with 50 and 100 μM ACR. XTT assays were performed to detect cell viability. Activities of superoxide dismutase (SOD), glutathione peroxidases (GSH-Px), and catalase (CAT), as well as total anti-oxidation competence (T-AOC) were examined. Superoxide anion levels were detected by a fluorescent probe. Cell ultrastructure changes were observed by transmission fluorescent microscope. Actin filament (F-actin) distribution was detected by immunofluorescence, and ERK and p38MAPK expression were detected by western blot analysis. ACR significantly decreased the viability of Sertoli cells in a dose- and time-dependent manner. T-AOC and the antioxidant activity of SOD, CAT and GSH-Px, were decreased in ACR-treated groups compared with the control group. The levels of reactive oxygen species (ROS) in ACR-treated Sertoli cells were increased. In addition, characteristics of cell apoptosis such as mitochondrial swelling, aggregated chromatin, condensed cytoplasm, nuclei splitting, and nuclei vacuolization were observed in ACR-treated cells. Furthermore, ACR-treatment also induced microfilament aggregation, marginalization and regionalization. The expression levels of ERK and p38MAPK were also increased in ACR-treated cells in a dose- and time-dependent manner. ACR, a major CP metabolite, impairs the cytoskeleton which is likely caused by induction of the oxidative stress response through up-regulation of ERK and p38MAPK expression.






Similar content being viewed by others
References
Tripathi DN, Jena GB (2008) Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 248:96–103
Charak BS, Gupta R, Mandrekar P et al (1990) Testicular dysfunction after cyclophosphamide-vincristine-procarbazine-prednisolone chemotherapy for advanced Hodgkin’s disease: a long-term follow-up study. Cancer 65:1903–1906
Kenney LB, Laufer MR, Grant FD et al (2001) High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 91:613–621
Trasler JM, Hales BF, Robaire B et al (1986) Chronic low dose cyclophosphamide treatment of adult male rats: effect on fertility, pregnancy outcome and progeny. Biol Reprod 34:275–283
Hoorweg-Nijman JJ, Delemarrevande-Wall HA, De Wall FC et al (1992) Cyclophosphamide-induced disturbance of gonadotropin secretion manifesting testicular damage. Acta Endocrinol 126:143–148
Das UB, Mallick M, Debnath JM et al (2002) Protective effect of ascorbic acid on cyclophosphamide-induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl 4:201–207
Ghosh D, Das UB, Ghosh S et al (2002) Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: a correlative study with testicular oxidative stress. Drug Chem Toxicol 25:281–292
Manda K, Bhatia AL (2003) Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol 19:367–372
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843
Griveau JF, Le Lannou D (1997) Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 20:61–69
Conte G, Milardi D, De Marinis L et al (1999) Reactive oxygen species in male infertility: review of literature and personal observations. Panminerva Med 41:45–53
Iuchi Y, Kaneko T, Matsuki S et al (2004) Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem Cell Biol 121:123–130
Maiorino M, Ursini F (2002) Oxidative stress, spermatogenesis and fertility. Biol Chem 383:591–597
Show MD, Anway MD, Folmer JS et al (2003) Reduced intratesticular testosterone concentration alters the polymerization state of the Sertoli cell intermediate filament cytoskeleton by degradation of Vimentin. Endocrinology 144:5530–5536
Tindall DJ, Rowley DR, Murthy L et al (1985) Structure and biochemistry of the Sertoli cell. Int Rev Cytol 94:127–149
Vogl AW, Vaid KS, Guttman JA (2009) The Sertoli cell cytoskeleton. Adv Exp Med Biol 636:186–211
Monsees TK, Franz M, Gebhardt S et al (2000) Sertoli cells as a target for reproductive hazards. Andrologia 32:239–246
de Jonge ME, Huitema AD, Rodenhuis S et al (2005) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 44:1135–1164
Kehrer JP, Biswal SS (2000) The molecular effects of acrolein. Toxicol Sci 57:6–15
Adams JD, Klaidman LK (1993) Acrolein-induced oxygen radical formation. Free Radic Biol Med 15:187–193
Mythili Y, Sudharsan PT, Selvakumar E et al (2004) Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact 30:13–19
Luo J, Shi Ri (2004) Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int 44:475–486
Luo J, Shi R (2004) Acrolein induces oxidative stress in brain mitochondria. Neurochem Int 46:443–452
Arumugam N, Sivakumar V, Thanislass J et al (1997) Effects of acrolein on rat liver antioxidant defense system. Indian J Exp Biol 35:1373–1374
Gurtoo HL, Hipkens JH, Sharma SD (1981) Role of glutathione in the metabolism-dependent toxicity and chemotherapy of cyclophosphamide. Cancer Res 41:3584–3591
Westlind A, Malmebo S, Johansson I et al (2001) Cloning and tissue distribution of a novel human cytochrome p450 of the CYP3A subfamily, CYP3A43. Biochem Biophys Res Commun 281:1349–1355
Clifton RJ, O’Donnell L, Robertson DM et al (2002) Pachytene spermatocytes in co-culture inhibit rat Sertoli cell synthesis of inhibin beta B-subunit and inhibin B but not the inhibin alpha-subunit. J Endocrinol 172:565–574
Galdieri M, Zani B (1981) Hormonal induced changes in Sertoli cell glycoproteins. Cell Biol Int Rep 5:111
Li LS, Li XL, Wei GH et al (2007) The oxidative stress impairment of immature Sertoli cells by acrolein. Chin J Pediatr Surg 28:318–321 (in Chinese)
Halliwell B, Gutteridge JMC (1998) Free radicals in biology and medicine, 3rd edn. Oxford Science, Oxford
Aitken J, Fisher H (1994) Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 16:259–267
Bauche F, Fouchard MH, Jegou B (1994) Antioxidant system in rat testicular cells. FEBS Lett 349:392–396
Tramer F, Rocco F, Micali F et al (1998) Antioxidant systems in rat epididymal spermatozoa. Biol Reprod 59:753–758
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Roy J, Pallepati P, Bettaieb A et al (2009) Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem Biol Interact 181:154–167
Angkeow P, Deshpande SS, Qi B et al (2002) Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ 9:717–725
Deshpande SS, Angkeow P, Huang J et al (2000) Racl inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASB J 14:1705–1714
Macho A, Hirsch T, Marzo I et al (1997) Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol 158:4612–4619
Guan X, Ruch RJ (1996) Gap junction endocytosis and lysosomal degradation of connexin43-P2 in WB-F344 rat liver epithelial cells treated with DDT and lindane. Carcinogenesis 17:1791–1798
Defamie N, Mograbi B, Roger C et al (2001) Disruption of gap junctional intercellular communication by lindane is associated with aberrant localization of connexin43 and zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis 22:1537–1542
Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G (2004) Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol 18(3):413–421
Calingasan NY, Uchida K, Gibson GE (1999) Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J Neurochem 72:751–756
Tirumalai R, Rajesh KT, Mai KH et al (2002) Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol Lett 132:27–36
Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185
Dalton TP, Shertzer HG, Puga A (1999) Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol 39:67–101
Kyriakis JM, Avruch J (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271:24313–24316
Li MW, Mruk DD, Lee WM et al (2009) Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41:2302–2314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Li, XL., Lin, T. et al. The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol Biol Rep 39, 493–500 (2012). https://doi.org/10.1007/s11033-011-0763-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0763-9