Abstract
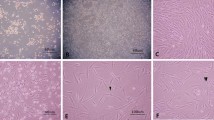
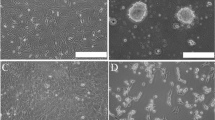
Nerve growth factor (NGF) is required for the differentiation and maintenance of sympathetic and sensory neurons. In the present study, the recombinant expression of human nerve growth factor beta (hNGF-β) gene in rabbit bone marrow mesenchymal stem cells (rMSCs) was undertaken. Recombinant vector containing hNGF-β was constructed and transferred into rMSCs, the expressions of the exogenous in rMSCs were determined by reverse transcriptase PCR (RT-PCR), ELISA and Western blot, whereas the biological activity of recombinant hNGF-β was confirmed using PC12 cells and cultures of dorsal root ganglion neurons from chicken embryos. The results showed that the hNGF-β gene expressed successfully in the rMSCs, a polypeptide with a molecular weight of 13.2 kDa was detected. The maximal expression level of recombinant hNGF-β in rMSCs reached 126.8012 pg/106 cells, the mean concentration was 96.4473 pg/106 cells. The recombinant hNGF-β in the rMSCs showed full biological activity when compared to commercial recombinant hNGF-β.





Similar content being viewed by others
Abbreviations
- NGF:
-
Nerve growth factor
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- MSCs:
-
Bone marrow mesenchymal stem cells
- RMSCs:
-
Rabbit mesenchymal stem cells
References
Levi-Montalcini R (1987) The nerve growth factor thirty-five years later. EMBO J 6(5):1145–1154
Thoenen H, Barde YA (1980) Physiology of nerve growth factor. Physiol Rev 60(4):1284–1335
Pizzo DP, Thal LJ (2004) Intraparenchymal nerve growth factor improves behavioral deficits while minimizing the adverse effects of intracerebroventricular delivery. Neuroscience 124(4):743–755
Muangman P, Muffley LA, Anthony JP, Spenny ML, Underwood RA, Olerud JE, Gibran NS (2004) Nerve growth factor accelerates wound healing in diabetic mice. Wound Repair Regen 12(1):44–52
Kawamoto K, Matsuda H (2004) Nerve growth factor and wound healing. Prog Brain Res 146:369–384
Mammoto T, Seerattan RA, Paulson KD, Leonard CA, Bray RC, Salo PT (2008) Nerve growth factor improves ligament healing. J Orthop Res 26(7):957–964
Conner JM, Fass-Holmes B, Varon S (1994) Changes in nerve growth factor immunoreactivity following entorhinal cortex lesions: possible molecular mechanism regulating cholinergic sprouting. J Comp Neurol 345(3):409–418
Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH (1987) Melioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329(6134):65–68
Fischer W (1994) Nerve growth factor reverses spatial memory impairments in aged rats. Neurochem Int 25(1):47–52
Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS (1994) Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci 14(8):4815–4824
Xiao B, Li QW, Feng B, Han ZS, Gao W, Li J, Li K, Zhao R, Jiang ZL, Hu JH, Zhi XB (2008) High-level expression of recombinant human nerve growth factor beta in milk of nontransgenic rabbits. J Biosci Bioeng 105(4):327–334
Jakubowska-Dogru E, Gumusbas U (2005) Chronic intracerebroventricular NGF administration improves working memory in young adult memory deficient rats. Neurosci Lett 382(1–2):45–50
Missale C, Spano P (1998) Nerve growth factor in pituitary development and pituitary tumors. Front Neuroendocrinol 19(2):128–150
Frade JM, Barde YA (1998) Nerve growth factor: two receptors, multiple functions. BioEssays 20(2):137–145
Rogers BC (1996) Development of recombinant human nerve growth factor (rhNGF) as a treatment for peripheral neuropathic disease. Neurotoxicology 17(3–4):865–870
Arevalo MA, Roldan PM, Chacón PJ, Rodríguez-Tebar A (2009) Amyloid beta serves as an NGF-like neurotrophic factor or acts as a NGF antagonist depending on its concentration. J Neurochem 111(6):1425–1433
Bernabei R, Landi F, Bonini S, Onder G, Lambiase A, Pola R, Aloe L (1999) Effect of topical application of nerve-growth factor on pressure ulcers. Lancet 354(9175):307
Tuveri M, Generini S, Matucci-Cerinic M, Aloe L (2000) NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet 356(9243):1739–1740
Lambiase A, Manni L, Rama P, Bonini S (2003) Clinical application of nerve growth factor on human corneal ulcer. Arch Ital Biol 141(2–3):141–148
Tan MH, Bryars J, Moore J (2006) Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea 25(3):352–355
Lambiase A, Coassin M, Sposato V, Micera A, Sacchetti M, Bonini S, Aloe L (2007) NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies. Pharmacol Res 56(1):65–69
Ting L, Bo W, Li R, Chen X, Wang Y, Jun Z, Yu L (2009) AMP-activated protein kinase supports the NGF-induced viability of human HeLa cells to glucose starvation. Mol Biol Rep [Epub ahead of print]
Fujimori K, Fukuzono S, Kotomura N, Kuno N, Shimizu N (1992) Overproduction of biologically-active human nerve growth factor in Escherichia coli. Biosci Biotechnol Biochem 56(12):1985–1990
Nishizawa M, Ozawa F, Higashizaki T, Hirai K, Hishinuma F (1993) Biologically active human and mouse nerve growth factors secreted by the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 38(5):624–630
Nguyen B, Jarnagin K, Williams S, Chan H, Barnett J (1993) Fed-batch culture of insect cells: a method to increase the yield of recombinant human nerve growth factor (rhNGF-β) in the baculovirus expression system. J Biotechnol 31:205–217
Bruce G, Heinrich G (1989) Production and characterization of biologically active recombinant human nerve growth factor. Neurobiol Aging 10(1):89–94
Iwane M, Kitamura Y, Kaisho Y, Yoshimura K, Shintani A, Sasada R, Nakagawa S, Kawahara K, Nakahama K, Kakinuma A (1990) Production, purification and characterization of biologically active recombinant human nerve growth factor. Biochem Biophys Res Commun 171(1):116–122
Schmelzer CH, Burton LE, Chan WP, Martin E, Gorman C, Canova-Davis E, Ling VT, Sliwkowski MB, McCray G, Briggs JA, Nguyen TH, Polastri G (1992) Biochemical characterization of recombinant human nerve growth factor. J Neurochem 59(5):1675–1683
Xiao B, Li Q, Feng B, Han Z, Gao D, Zhao R, Li J, Li K, Zhi X, Yang H, Liu Z (2009) Expression of recombinant human nerve growth factor beta in milk of goats by recombinant replication-defective adenovirus. Appl Biochem Biotechnol 157(3):357–366
Coulibaly S, Besenfelder U, Fleischmann M, Zinovieva N, Grossmann A, Wozny M, Bartke I, Togel M, Muller M, Brem G (1999) Human nerve growth factor beta (hNGF-beta): mammary gland specific expression and production in transgenic rabbits. FEBS Lett 444(1):111–116
Werner RG (1998) Innovative and economic potential of mammalian cell culture. Arzneimittelforschung 48(4):423–426
Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19(3):180–192
Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 28(8):875–884
Minguell JJ, Erices A, Conget P (2001) Mesenchymal stem cells. Exp Biol Med 226(6):507–520
Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ (1995) Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci 92(11):4857–4861
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164(2):247–256
Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z (2009) AMP-activated protein kinase supports the NGF-induced viability of human HeLa cells to glucose starvation. Mol Biol Rep 36(4):725–731
Shakhbazau A, Shcharbin D, Seviaryn I, Goncharova N, Kosmacheva S, Potapnev M, Gabara B, Ionov M, Bryszewska M (2009) Use of polyamidoamine dendrimers to engineer BDNF-producing human mesenchymal stem cells. Mol Biol Rep [Epub ahead of print]
Hou X, Wu X, Ma J, Lv X, Jin X (2010) Erythropoietin augments the efficacy of therapeutic angiogenesis induced by allogenic bone marrow stromal cells in a rat model of limb ischemia. Mol Biol Rep 37(3):1467–12475
Chung N, Jee BK, Chae SW, Jeon YW, Lee KH, Rha HK (2009) HOX gene analysis of endothelial cell differentiation in human bone marrow-derived mesenchymal stem cells. Mol Biol Rep 36(2):227–235
Liu H, Fan H, Toh SL, Goh JC (2008) A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials 29(10):1443–1453
Jin B, Luo XP, Ni HC, Li Y, Shi HM (2009). Cardiac matrix remodeling following intracoronary cell transplantation in dilated cardiomyopathic rabbits. Mol Biol Rep [Epub ahead of print]
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press
Omoto M, Miyashita H, Shimmura S, Higa K, Kawakita T, Yoshida S, McGrogan M, Shimazaki J, Tsubota K (2009) The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci 50(5):2109–2115
Scott J, Selby M, Urdea M, Quiroga M, Bell GI, Rutter WJ (1983) Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature 302(5908):538–540
Stoeltzing O, Ahmad SA, Liu W, McCarty MF, Parikh AA, Fan F, Reinmuth N, Bucana CD, Ellis LM (2002) Angiopoietin-1 inhibits tumour growth and ascites formation in a murine model of peritoneal carcinomatosis. Br J Cancer 87(10):1182–1187
Yu BF, Li WI, Hu XN, Zhang YH, Niu B, Xie J (2009) Hepatocyte gene transfer mediated by stable polyplexes based on MPP-containing DNA complexes. Hepatobiliary Pancreat Dis Int 8(5):498–503
Fang R, Nie H, Wang Z, Tu P, Zhou D, Wang L, He L, Zhou Y, Zhao J (2009) Protective immune response in BALB/c mice induced by a suicidal DNA vaccine of the MIC3 gene of Toxoplasma gondii. Vet Parasitol 164(2–4):134–140
Jiang Y, Wang Y, Kuang Y, Wang B, Li W, Gong T, Jiang Z, Yang D, Li M (2009) Expression of mouse beta-defensin-3 in MDCK cells and its anti-influenza-virus activity. Arch Virol 154(4):639–647
Kano FS, Tamekuni K, Coelho AL, Garcia JL, Vidotto O, Itano EN, Vidotto MC (2008) Induced immune response of DNA vaccine encoding an association MSP1a, MSP1b, and MSP5 antigens of Anaplasma marginale. Vaccine 26(27–28):3522–3527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, BS., Lou, JY. Recombinant expression of human nerve growth factor beta in rabbit bone marrow mesenchymal stem cells. Mol Biol Rep 37, 4083–4090 (2010). https://doi.org/10.1007/s11033-010-0068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0068-4