Abstract
Acylsugars are important insect defense compounds produced at high levels by glandular trichomes of the wild tomato, Solanum pennellii. The ability to produce acylsugars at elevated levels was bred into the tomato line CU071026. This study utilized a marker-assisted backcross approach to individually introgress into CU071026 and to fine map the three quantitative trait loci (QTL) fatty acid 5 (FA5QTL), fatty acid 7 (FA7QTL), and fatty acid 8 (FA8QTL), which were previously associated with changes in acylsugar chemistry. Additional breeding with and fine mapping the previously introgressed QTL, fatty acid 2 (FA2QTL), was also conducted. The effect of these four QTL on acylsugar quality and quantity in the presence of the five introgressions of CU071026 was evaluated. Incorporation of the QTL altered acylsugar chemotype by modulating the length, orientation, and/or relative proportion of fatty acid acyl groups. The resulting quantities of acylsugar produced in most of the new lines were similar to that of CU071026; however, introgression of FA5QTL reduced acylsugar levels. The acylsugar lines containing each QTL were characterized for acylsugar level, trichome abundance, and acylsugar chemistry through gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry. The novel acylsugar chemotype lines created can contribute to elucidation of the mechanism of insect resistance mediated by acylsugars and help with identification of yet-unknown genes contributing to acylsugar synthesis and diversity.
Similar content being viewed by others
Introduction
Human population growth, dietary needs, and a more variable climate demand increased, sustainable food production. Phytophagous insects and the viruses they vector are among the most significant challenges to maintaining and increasing crop quality and yield. Plants have evolved in myriad ways to combat threats from herbivores and pathogens, including the production of specialized metabolites that facilitate mediation of physiological stresses and also function as defenses. Breeding for metabolites that contribute to plant defense has not been a major focus of plant breeders since use of synthetic pesticides provided cheap and relatively effective control of insects. However, increased awareness of economic and environmental costs of chemical control and the desire to support sustainable agriculture demand novel plant breeding strategies including utilization of natural defensive traits to control plant pests. The tremendous diversity of secondary metabolites implies ecological functions in stress mediation (Leckie et al. 2016) and provides opportunities and valuable tools to employ plant chemistry to improve host-plant resistance and advance food security (Dixon 2001; Schilmiller et al. 2008; Wink 2010).
One promising class of secondary metabolites receiving increased attention is a family of sugar polyesters known as acylsugars. Acylsugars are accumulated by numerous species in the nightshade family (Solanaceae), such as wild potato (Solanum berthaultii), tobacco (Nicotiana tabacum), Petunia (Petunia hybrida), and several wild relatives of tomato, such as Solanum pennellii, S olanum galapagense, and Solanum habrochaites (Fobes et al. 1985; Burke et al. 1987; King et al. 1986, 1988; King and Calhoun 1988; Ohya et al. 1996; Kim et al. 2012; Schilmiller et al. 2015). Acylsugars are implicated in mediating a variety of plant–insect interactions, including feeding deterrence and oviposition preference (Severson et al. 1985, Goffreda and Mutschler 1989; Hawthorne et al. 1992; Rodriguez et al. 1993; Juvik et al. 1994, Liedl et al. 1995, Fancelli et al. 2005; Leckie et al. 2016). In Nicotiana attenuata, acylsugars play a role in indirect defense against Manduca sexta by serving as a volatile attractant to a ground hunting ant, Pogonomyrmex rugosus, after undergoing hydrolysis in the caterpillar midgut (Weinhold and Baldwin 2011). In tomato species, the acylsugars produced and secreted from glandular trichomes are composed of a sugar backbone (sucrose or glucose) to which several short to medium chain aliphatic acids are esterified. These fatty acids can be either straight-chained or branched (Fobes et al. 1985; Burke et al. 1987; Shapiro et al. 1994; Schilmiller et al. 2010, 2012, 2016; Fan et al. 2016).
S. pennellii (Correll) D’Arcy accession LA716 accumulates substantial amounts of acylsugars which have been shown to effectively control many insects and provides a promising source of direct insect resistance (Goffreda and Mutschler 1989; Hawthorne et al. 1992; Rodriguez et al. 1993; Juvik et al. 1994; Shapiro et al. 1994; Liedl et al. 1995) that can be transferred to tomato (Mutschler and Wintermantel 2006, Leckie et al. 2012). Efforts to transfer acylsugar production from S. pennellii LA716 into tomato led to the creation of the Cornell benchmark line, CU071026, which accumulates ca. 15 % the level of acylsugar of S. pennellii LA716 (Leckie et al. 2012). CU071026 contains five introgressions from LA716 on chromosomes 2, 3, 7, and 10 (called AS2, AS3, AS7, AS10.1, and AS10.2, respectively; see Supplementary Table S1 of Leckie et al. 2012 for markers and map positions of the S. pennellii LA716 introgressions in CU071026 and Supplementary Fig. S1 of this paper for a visual depiction of the introgressions). Although the level of acylsugars accumulated by CU071026 and its sister lines is lower than the extremely high levels of S. pennellii LA716, the level of acylsugars produced by these tomato lines is sufficient to significantly reduce Bemisia tabaci oviposition on lines grown in field cages (Leckie et al. 2012) and acylsugar-producing hybrids also reduced the incidence of tomato infectious chlorosis virus in fields with heavy pressure from the whitefly Trialeurodes vaporariorum (Mutschler and Wintermantel 2006).
S. pennellii accessions have various acylsugar chemotypes that vary with geographical location (Shapiro et al. 1994; Ning et al. 2015), which suggests the possibility of adaptation and selection of specific metabolic profiles in response to local herbivore pressures. The acylsugars of S. pennellii LA716 are predominantly acylglucoses with a characteristic array of fatty acids including 2-methylpropanoate (iso branched 4 carbon acyl group) (i-C4), 2-methylbutanoate (anteiso branched 5 carbon acyl group) (ai-C5), 3-methylbutanoate (iso branched 5 carbon acyl group) (i-C5), 8-methylnonanoate (iso branched 10 carbon acyl group) (i-C10), n-decanoate (straight chain 10 carbon acyl group) (n-C10), and n-dodecanoate (straight chain 12 carbon acyl group) (n-C12) (Burke et al. 1987; Shapiro et al. 1994; Blauth et al. 1999). In contrast, the profile of CU071026, which was bred using S. pennellii LA716, is almost exclusively acylsucroses with predominantly ai-C5, i-C5, and n-C12 fatty acids and only trace or undetectable levels of i-C4, i-C10, and n-C10 (Leckie et al. 2014). The fatty acid profile of CU071026 is similar to that of cultivated tomato, which predominantly accumulates i-C5, ai-C5, and n-C12 fatty acids as well (Schilmiller et al. 2010; Ghosh et al. 2014). Work with purified acylsugars from CU071026 and several S. pennellii accessions, including S. pennellii LA716, indicates that purified acylsugars of CU071026 are less effective at equimolar levels than purified acylsugars of some S. pennellii accessions at controlling whitefly (B. tabaci) and western flower thrips (Frankliniella occidentalis) feeding and oviposition in laboratory assays (Leckie et al. 2016), suggesting that insect control of CU071026 or derived lines could be improved by altering their acylsugar chemotypes. Quantitative trait loci (QTL) that affect acylsugar chemistry have been identified (Blauth et al. 1998, 1999; Schilmiller et al. 2010, 2012, 2015; Leckie et al. 2013, 2014; Fan et al. 2016) and shown to alter the chemotype of acylsugars accumulated in tomato lines (Schilmiller et al. 2010) such as the mono-introgression lines (ILs) created by Eshed and Zamir (1994, 1995). Addition of QTL that alter acylsugar chemotype into CU071026 could provide a means of generating acylsugars with stronger or broader insect resistance than that of CU071026.
The objectives of this study were to individually introgress several previously identified acylsugar chemotype QTL into CU071026 to create a set of tomato sister lines and extensively characterize these lines for alterations in the acylsugars accumulated through gas chromatography mass spectrometry (GC-MS) and liquid chromatography mass spectrometry (LC-MS). The ILs were identified as an optimum source of the desired S. pennellii LA716 acylsugar chemotype QTL for transfer to CU071026. Three QTL, fatty acid 5 (FA5QTL) and fatty acid 7 (FA7QTL) (identified in Leckie et al. 2014) and fatty acid 8 (FA8QTL) (identified in Blauth et al. 1999; Schilmiller et al. 2010; Leckie et al. 2014), previously shown to alter acylsugar chemistry, were introgressed into CU071026 to test the effect of these QTL in an acylsugar-producing tomato line, both to confirm function and to create tomato lines for testing against insects. Identification of plants with recombinations within these introgressions during transfer allowed fine mapping of the QTL within them. An additional QTL, fatty acid 2 (FA2QTL), on chromosome 2, was previously introgressed into CU071026 (Leckie et al. 2014); additional selection was performed to create a FA2 acylsugar line (FA2/AS). The acylsugars accumulated by the altered acylsugar chemotype lines were characterized by acylsugar assay, which measures acylsugar level, by GC-MS analysis, which determines relative proportions of the fatty acids present, and by LC-MS characterization, which determines the relative proportions of acylsugar molecules accumulated with information concerning the number and length of fatty acids esterified to the sugar backbone. The implications of these data are discussed, including whether adding the acylsugar chemotype QTL affects levels of acylsugars accumulated, leads to greater diversity in the fatty acids esterified to the sugar molecules, and/or also in changes in the specific acylsugars accumulated.
Materials and methods
Plant materials
CU071026 is an acylsugar-producing tomato line bred using S. pennellii LA716 by the Cornell University tomato breeding program, which is the source of the seed used. Seeds from S. pennellii LA716 were produced by the Cornell University tomato breeding program, derived from seed originally obtained from the Tomato Genetics Resource Center (TGRC) at the University of California at Davis. The modified acylsugar tomato line FA2/AS was developed from CU071026 by the Cornell University tomato breeding program, which is the source of FA2/AS seed.
A series of tomato lines with individual introgressions of S. pennellii LA716 DNA in the processing tomato M82 (a sub-selection of UC82-B) were produced by Eshed and Zamir (1994, 1995). Based on prior QTL analysis, the introgression line IL2-4 was used by the Cornell University tomato breeding program as the source of FA2QTL (Leckie et al. 2014). Similarly, we chose the introgression lines IL5-3, IL7-4-1, and IL8-1-1 to use as sources of the FA5, FA7, and FA8 QTL for transfer. The seed of IL5-3, IL7-4-1, and IL8-1-1 was produced at Cornell University, derived from seed obtained from D. Zamir (Hebrew University of Jerusalem, Rehovot, Israel). Seed of M82 was produced by the Cornell University tomato breeding program, derived from seed originally obtained from the Tomato Genetics Resource Center (TGRC) at the University of California at Davis.
Plant growth conditions
Seeds were germinated in 32-cell flat cups with LM1 (Lambert, Rivière-Ouelle, Quebec, Canada) mix until ca. 5 weeks of age, during which time any necessary marker-based genetic analysis could be completed. Selected plants were transplanted to 8-in. clay pots of LM111 (Lambert, Rivière-Ouelle, Quebec, Canada) mixed with turface (Turface Athletics, Buffalo Grove, IL) in a 1:1.8 ratio, with 0.3 % unimix (10–5–10) and calcium sulfate additive. Plants for all populations and experiments were grown in a greenhouse in the Guterman Bioclimatic Laboratory and Greenhouse Complex at Cornell University in Ithaca, NY and were typically maintained at 29 °C:20 °C day night temperatures with a 16:8-h light/dark photoperiod.
Breeding scheme for transfer of QTL
For transfer of each QTL, an IL line was selected that putatively contained the QTL of interest. Selection of IL line was based on marker data from IL lines compared to QTL marker mapping intervals identified in Leckie et al. (2014). The selected IL line was crossed as the female parent to CU071026 and the resulting F1 plant backcrossed to CU071026 to create the BC1F1 populations (CU071026 × (IL line × CU071026). Selection of plants from the BC1F1 populations was based on markers within the five S. pennellii LA716 introgressions possessed by CU071026 and markers within the additional introgression being introduced into CU071026. The original introgressions of CU071026 were selected for homozygosity, using markers, to maintain the CU071026 introgressions in the new line. The markers within the new IL introgression were utilized to select for both presence of the new introgressions, and for plants with recombinations within the new introgressions, to reduce introgression sizes and fine map the new QTL within these introgressions.
Genotypic screening
Molecular markers utilized in all populations to select for CU071026 regions are provided in Supplementary Table S1. Identity and location of markers used to introgress FA5QTL, FA7QTL, and FA8QTL into the presence of the five CU071026 introgressions are provided in Supplementary Table S2.
Phenotypic screening
Acylsugar level
Levels of acylsugar for plants of the controls and of populations in the development of the FA/AS lines were measured on 9–10 weeks of age plants using the method of Leckie et al. (2012), which is a modification of the prior method described by Goffreda et al. (1990), replacing the Nelson reaction originally used to measure sugar (Goffreda et al. 1990) with a modified peroxidase/glucose oxidase assay (Setter et al. 2001) that measures glucose. For these replicated screens, four plants of each genotype were sampled, collecting four samples of two lateral leaflets from leaves that were two to three nodes from the apex of stems. Each two leaflet sample was placed in wide mouth plastic scintillation vials and completely dried in a seed dryer at 29 °C. Fully dried leaflets were rinsed with 3 ml of methanol containing methyl heptanoate (30 mg L-1), an internal standard for fatty acid analysis. The assay uses 100 µl of each rinsate. Leaflets were redried immediately after rinsing and weighed, so that acylsugar level could be expressed per weight dried leaf. Dried leaf weights ranged from about 50 to 90 mg. Acylsugar level data were analyzed using ANOVA in JMP Pro 11 (SAS Institute Inc. 2014), and means were separated by Tukey-Kramer HSD (p < 0.05). Prior to analysis, acylsugar level data were Ln(x) transformed to improve normality.
Fatty acid characterization
Percentages of each type of fatty acids from each sample were ascertained by collecting pairs of young, fully expanded primary lateral leaflets, rinsing leaflets with 3 ml of methanol containing methyl heptanoate (30 mg L-1) as an internal standard, and then utilizing transmethylation/GC-MS analysis, as described in Leckie et al. (2014). Peak areas of the resulting chromatograms were calculated using Varian MS Workstation Version 6.9.1 (Agilent Technologies, Santa Clara, CA), and levels of respective fatty acids were determined through comparison with levels of the internal standard to generate relative proportions of each fatty acid. Percent fatty acid GC data was analyzed using ANOVA in JMP Pro 11 (SAS Institute.JMP®, Version 11. SAS Institute Inc., Cary, NC, pp. 1989-2007) and means separated by Tukey-Kramer HSD (p < 0.05). Prior to analysis, data for i-C4, n-C10, and 9-methyldecanoate (i-C11) aliphatic acids were cube root transformed and the data for ai-C5 and 11-methyldodecanoate (i-C13) were log10(x + 1) transformed to improve normality.
Heritability estimation
Broad sense heritability estimates were generated by using acylsugar level and fatty acid data from 2014 and 2015 over several environments. Heritability for acylsugar level and the major fatty acids was calculated according to Holland et al. (2003) using variances obtained from the lmer function in the lme4 package in R (Bates et al. 2014) where genotype, location, year, genotype by location, and genotype by year were treated as random effects.
Acylsugar structure characterization
LC-MS was utilized to analyze the structures of acylsugars accumulated in each line. Three samples of a single primary lateral leaflet per genotype were taken and extracted with a buffer consisting of isopropanol/acetonitrile/water (3:3:2 v/v/v) containing 0.1 % formic acid and 10 µM of propyl-4-hydroxybenzoate, an internal standard, and processed as described in Schilmiller et al. (2015). Acylsugar results from the LC-MS analysis are described using the nomenclature of Schilmiller et al. (2010), in which the acylsugar name S4:17 indicates a sucrose backbone sugar, with four fatty acid acyl chains that have a total of 17 carbons. LC-MS data were analyzed by hierarchical clustering with a Pearson correlation using a pairwise average-linkage clustering method for both genotypes and acylsugars using the hierarchical clustering tools provided by GenePattern Reich et al. (2006).
Trichome density
Since acylsugars are secreted by glandular trichomes, the density of type IV and type VI trichomes was evaluated for each fatty acid line to provide further characterization of factors related to acylsugar production and defense. Four plants of each fatty acid line were simultaneously grown in a greenhouse and sampled at 9–10 weeks of age for trichome counts. Two young, expanding, primary lateral leaf samples were taken from each plant; two interveinal areas on the abaxial side of the leaflet from each sample were used to count trichomes. Trichomes were visualized with a Carl Zeiss 475003, 9901 microscope using 63x power. An eye piece grid was utilized to facilitate counting, and a 5 × 5 section of the grid, where each square measured 0.0256 mm2, was used to count trichomes, for a total counting area of 0.64 mm2. Only those trichomes whose base fell in the 5 × 5 grid were counted. Trichome counts were later adjusted to number of trichomes per square millimeter and trichome density data was analyzed using ANOVA in JMP Pro 11 (SAS Institute Inc. 2014) and means separated by Tukey-Kramer HSD (p < 0.05). Prior to analysis, data for density of type IV and VI trichomes were log10(x + 1) transformed to improve normality.
Results and discussion
Introgressing FA7QTL
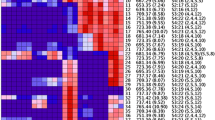
FA7QTL was readily transferred into CU071026 and fine mapped due to the dominant nature of this QTL. FA7QTL was putatively localized in and introgressed from IL7-4-1, which possesses a 56.3-Mbp chromosome 7 introgression from S. pennellii LA716 in the M82 background (Long et al. 2013). A 380 individual (CU071026 × (CU071026 × IL7–4-1)) BC1F1 population was used to identify, through marker-assisted selection (MAS), those plants that were homozygous for the majority of the five CU071026 S. pennellii introgressed regions and were also heterozygous for the IL7-4-1 introgression. In addition, one recombinant BC1F1 plant (131285-176) was identified that was homozygous for the CU071026 introgressions AS3, AS10.1, and AS10.2 and heterozygous for the CU071026 introgressions AS2 and AS7, as well as heterozygous for a ca. 3.2-Mb subregion of the IL7-4-1 introgression. BC1F1 plant 131285-176 and sibling plants containing the entire IL7-4-1 introgression and homozygous for AS2 accumulated acylsugar levels at least as high as that of the CU071026 control and selections homozygous for AS2 but lacking the entire IL7-4-1 introgression (data not shown). Additionally, 131285-176 and plants containing the entire IL7-4-1 region produced acylsugars with an increase in n-C10 and a decrease in n-C12 fatty acids compared to selections lacking the IL7-4-1 introgression (Fig. 1) which is consistent with the impact of FA7QTL on acylsugar fatty acid profile as described by Leckie et al. (2014). Specifically, BC1F1 plants that did not possess the IL7-4-1 introgression averaged 1.2 % n-C10 and 45.6 % n-C12, while BC1F1 plants possessing the IL7-4-1 introgression accumulated on average 5.3 % n-C10 and 38.0 % n-C12. The GC fatty acid profile of these BC1F1 plants confirms that the full IL7-4-1 introgression contains FA7QTL, that the subintrogression in 131285-176 still possesses FA7QTL, and that FA7QTL functions in a background that contains the five introgressions of CU071026. These results also support those of Leckie et al. (2014), which indicate that FA7QTL reduces the extension fatty acid products by two carbons. The level of acylsugars accumulated was not compared among the selections because the CU071026 introgressions (AS2 and AS3) are recessive and were segregating, thus confounding any effect of the IL7-4-1 introgression on acylsugar level in this generation.
Markers, genotypes, and selected fatty acid data, diagnostic for the presence of FA7QTL, in CU071026 and selected individuals out of a BC1F1 population showing the relative location of FA7QTL as past snp_100893 (solcap_snp_sl_100893) and tightly linked to C2_At2g30520. N-C10 fatty acid cube root transformed prior to analysis. Almost equal to sign represents a large physical distance. Means followed by different letters within a column are significantly different at P < 0.05
The purpose of the BC1F2 population was to obtain plants homozygous for FA7QTL and to observe the effect of this QTL in the homozygous condition in an acylsugar background, which has not previously been reported. The 131285-176 individual that was homozygous for the AS3 and AS10 CU071026 introgressions and heterozygous for the AS2 and AS7 introgressions of CU071026 was chosen for production of a BC1F2 population. In this BC1F1 selection, the AS7 introgression and sub-IL7-4-1 introgression were in trans configuration, so a recombination between them was necessary to get both regions homozygous in the BC1F2. Individuals from a 188 plant BC1F2 population were selected by MAS and grown to obtain plants that were homozygous for the AS2 and AS7 CU071026 regions as well as for the ca. 3.2 Mbp subsection of the IL7-4-1 introgression. We screened for plants in which both of the parental gametes were recombinant between AS7 and FA7 so that the plants were homozygous for both AS7 and the IL7-4-1 subregion; five of the 188 plants had the desired recombinations so that the AS7 and sub-IL7-4-1 regions were both homozygous and 2 of these plants were also homozygous for AS2. These two individuals, as well as a plant that was homozygous for the AS2 and sub-IL7-4-1 regions but had lost AS7, all produced high levels of acylsugar and accumulated increased n-C10 and decreased n-C12 fatty acids, which suggests the AS7 introgression is not necessary to maintain acylsugar level and has no impact on the fatty acid profile (Table 1). The GC fatty acid profile of tomato plants homozygous for the S. pennellii LA716 introgressions within CU071026 and also for the ca. 3.2 Mbp sub-IL7-4-1 region is largely the same for plants heterozygous for the entire or sub-IL7-4-1 introgression, which suggests that FA7QTL is largely dominant in its impacts (Fig. 1 and Table 1). The two plants homozygous for the ca. 3.2 Mbp FA7QTL subregion containing both AS7 and FA7QTL as well as for the other four introgressions of CU071026 were observed in the greenhouse and one with higher seed set was selected as the initial plant to establish the line FA7/AS. A depiction of the S. pennellii LA716 introgressions in the FA7/AS line is found in Supplementary Fig. S1.
GC data from additional recombinant plants in FA7QTL BC1F1 fine mapped the location of FA7QTL within the original ca. 56.3 Mbp IL7-4-1 introgression and facilitated selection of a ca. 3.2 Mbp subregion carrying FA7QTL. All recombinant plants that were heterozygous for at least marker C2_At2g30520 (Tomato SL2.50 ITAG2.4 Solgenomics.net) (56,723,010–56,725,124 bp), near the bottom of the IL7-4-1 introgression, accumulated acylsugar with increased n-C10 (Fig. 1). Three recombinant plants that were heterozygous for most of the introgression, but that had lost the region near C2_At2g30520, accumulated low levels of n-C10 and higher levels of n-C12; averages of 1.4 and 47.7 %, respectively. Together these data indicated FA7QTL was tightly linked with the C2_At2g30520 marker and that FA7QTL was between dCAPS_100893 (derived from solcap_snp_sl_100893) (ca. 54,043,669 – 54,044,558) and at or extending slightly past C2_At2g30520. Later genotyping utilizing genotyping by sequencing indicated the sub-FA7QTL introgression ranged from a single nucleotide polymorphism (snp) at 55,977,484 bp to a snp at 59,210,920 bp or ca. 3.2 Mbp in length.
Introgressing FA8QTL
Introgressing FA8QTL was more challenging due to the recessive nature of this QTL. FA8QTL was putatively localized in and transferred from IL8-1-1 which possesses a ca. 50 Mbp chromosome 8 introgression from S. pennellii LA716 (Long et al. 2013). A 184 individual BC1F1 population (CU071026 × (CU071026 × IL8-1-1)) was used to identify, through MAS, plants homozygous for the CU071026 regions and heterozygous for the IL8-1-1 introgression. While the BC1F1 population provided a number of recombinants for the IL8-1-1 introgression, we could not use GC data from BC1F1 plants to select recombinants that possessed FA8QTL, since the location of FA8QTL within the IL8-1-1 introgression was unknown and the effect of FA8QTL on the GC profile largely recessive (Leckie et al. 2014). Therefore, to ensure maintenance of FA8QTL, we selected a plant, 121225-111, which was heterozygous for the full length IL8-1-1 introgression and homozygous for all five CU071026 regions, to create a BC1F2 population and test QTL impact.
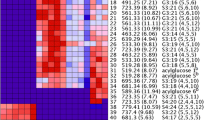
The purpose of the BC1F2 populations was to confirm that FA8QTL was contained within IL8-1-1 and observe its effect on acylsugar level and fatty acid profile in the presence of the five CU071026 introgressions. Seeds from the BC1F1 selection, 121225-111, were used to create a 67 plant FA8QTL BC1F2 (A) population, from which 16 plants were identified that were homozygous for the entire IL8-1-1 introgression. The five of those homozygous IL8-1-1 selections that were tested by GC analysis accumulated an average of 21.2 % i-C4 and 12.0 % i-C5 fatty acids (Fig. 2). Conversely, CU071026 accumulated 1.4 % i-C4 and 36.6 % i-C5; similarly, four BC1F2 (A) plants that lost the IL8-1-1 region accumulated 1.2 % i-C4 and 35.0 % i-C5. These results demonstrate the principle effect of FA8QTL, which is a large increase in i-C4 and a simultaneous decrease in i-C5 fatty acids. Four plants heterozygous for the IL8-1-1 introgression accumulated 1.9 % i-C4 and 33.8 % i-C5 fatty acids, further confirming the function of FA8QTL is largely recessive. BC1F2 (A) selections homozygous for the full length FA8QTL introgression appeared to accumulate slightly lower levels of acylsugars than that of CU071026 (Fig. 2), which could either be due to linkage drag in the ca. 50 Mbp IL8-1-1 introgression or to the impact of the QTL itself. Data from a BC1F2 (A) population confirm that IL8-1-1 contains FA8QTL and that it functions in the presence of the five CU071026 introgressions.
Markers, genotypes, and selected fatty acid data, diagnostic for the presence of FA8QTL, in CU071026 and selected individuals out of a BC1F2 population showing the relative location of FA8QTL between U221657 and snp_51969 (solcap_snp_sl_51969). Two additional markers run on all plants (C2_ At5g27390 at 22.8 Mbp and C2_ At1g30360 at 27.9 Mbp) revealed no recombinations between snp_51969 and C2_At4g33030. i-C4 data cube root transformed prior to analysis to improve normality. Almost equal to sign represents a large physical distance. Means followed by different letters within a column are significantly different at P < 0.05
Recombinant plants from the BC1F2 (A) population also allowed mapping of FA8QTL to a ca. 1.5 Mbp region from a couple recombinant plants (Fig. 2). The average i-C4 and i-C5 fatty acid proportion for recombinant plants 131095-040 and 131095-066 was 1.9 and 38.2 %, respectively, which was very similar to individuals heterozygous for the entire introgression, indicating FA8QTL was located in the heterozygous region both plants shared. This shared heterozygous region containing FA8QTL was ca. 1.5 Mbp from marker U221657 (3,280,372–3,283,363 bp) to solcap_snp_sl_51969 (4,744,567–4,744,601 bp) (Tomato SL2.50 ITAG2.4 Solgenomics.net). This location is consistent with the QTL on chromosome 8 identified in Blauth et al. (1999) for presence of iso-C4 fatty acids in acylsugars. One of the BC1F2 (A) individuals homozygous for the entire introgression that also set seed well in the greenhouse was selected to establish the new line designated FA8/AS. A depiction of the S. pennellii LA716 introgressions in the FA8/AS line is found in Supplementary Fig. S1.
Recent work by Ning et al. (2015) identified a nonfunctional isopropylmalate synthase (Solyc08g014230) in the S. pennellii LA716 introgression contained in the IL8-1-1 line and demonstrated that this gene is responsible for an increase in i-C4 fatty acids in the acylsugars of this IL line. This gene, originally integral in leucine biosynthesis, is critical for production of i-C5 Coenzyme A (CoA), a precursor of i-C5 fatty acids. A nonfunctional copy of this enzyme leads to an increase in i-C4 fatty acids, through an increase in i-C4 CoA, the precursor of i-C4 fatty acids. The location of Solyc08g014230 (Tomato SL2.50 ITAG2.4 Solgenomics.net) (3,945,013–3,955,435 bp) falls within our proposed region for FA8QTL and is likely the gene responsible for this QTL.
With the success in fine mapping FA8QTL, we attempted to use this information to decrease the size of the IL8-1-1 introgression in an acylsugar breeding line. Because neither recombinant plant from FA8QTL BC1F2 (A) had the type of subintrogression needed to produce the desired plant, we returned to a recombinant individual, 121225-31, from FA8QTL BC1F1 which had a recombination such that it had lost at least 40 Mbp of the IL8-1-1 introgression but still possessed the top ca. 6 Mbp of the IL8-1-1 introgression with FA8QTL. Plant 121225-31 was still heterozygous for the AS3 and AS10.1 regions of CU071026. Seeds from 121225-31 were sown to generate a 377 individual FA8QTL BC1F2 (B) population. Through MAS, we identified individuals that were homozygous for both CU071026 regions, but we observed extreme segregation distortion in this population for the sub-IL8-1-1 region, specifically 282 plants heterozygous for the sub-IL8-1-1 region, 92 plants that lost the region, and a complete lack of plants homozygous for the S. pennellii copy of the sub-IL8-1-1 region. This distortion was contrary to the segregation we observed in the BC1F2 (A) population, where the full length IL8-1-1 region segregated normally; however, it is consistent with a segregation distortion trait previously noted for chromosome 8 from S. pennellii (Eshed and Zamir 1994). These results suggest that some genetic characteristics of BC1F1 plant 121225-111, which was the parent of FA8QTL BC1F2 (A) population, allowed the normal segregation of full IL8-1-1 chromosome 8 introgression in this population, but the genetic characteristic was not present in BC1F1 plant 121225-31, the parent of FA8QTL BC1F2 (B) population. This pattern is further supported by results of additional progenies derived from BC1F2 (B) population. The self-progeny of one BC1F2 (B) plant (131254-066), which was homozygous for the CU071026 regions and heterozygous for only the sub-IL8-1-1 region, produced a 189 plant BC1F3 population with extreme distortion for the sub-IL8-1-1 region (54 plants homozygous for tomato alleles, 134 heterozygous, but no plants homozygous for S. pennellii alleles within the sub-IL8-1-1 region). Furthermore, a BC1F3 plant (131544-003) heterozygous for the IL8-1-1 subregion, and homozygous for all CU071026 regions, was crossed as a female to a line with the full length IL8-1-1 introgression selection from the FA8QTL BC1F2 (A) population. In this cross, both parental plants were homozygous for the CU071026 regions; therefore, the only S. pennellii LA716 region segregating was the sub-IL8-1-1 introgression. The 64 plant F1 progeny from this cross also showed extreme distortion, such that all individuals were found to be heterozygous for the sub-IL8-1-1 region, with a complete lack of individuals homozygous for S. pennellii LA716 from IL8-1-1.
In creating the IL lines, Eshed and Zamir (1994) noted the male gametes possessing the chromosome 8 S. pennellii LA716 alleles were eliminated in the original line IL8-1; the original introgression in line IL8-1 was maintained by selecting and self-pollinating plants heterozygous for the introgression. Later, line IL8-1-1 was released, in which the chromosome 8 introgression was homozygous and which was used in our work. It was initially thought that the IL8-1-1 introgression was reduced in size from that of IL8-1, perhaps eliminating a region responsible for the segregation distortion. However, more recent characterization by SSR markers indicates that IL8-1 and IL8-1-1 contain the same length S. pennellii LA716 introgression for chromosome 8 (Long et al. 2013). Additionally, recent genotyping of the original Zamir ILs revealed that IL8-1 and IL8-1-1 were identical for 3503 snps and in particular shared 85 snps in common on chromosome 8 from S. pennellii LA716 (Sim et al. 2012). The fact that IL8-1-1 introgression can be homozygous but is putatively the same length as IL8-1, which cannot be homozygous, might suggest the line IL8-1-1 contains a gene elsewhere in its genome which is necessary to allow normal segregation and homozygosity for the IL8-1/IL8-1-1 introgression. The loss of the normal segregation in the BC1F2 population (B) that was derived from IL8-1-1 could suggest that the factor is not within the region of the IL8-1-1 introgression.
Introgressing FA5QTL
The transfer of FA5QTL led to complex results due to its interaction with acylsugar level. Schilmiller et al. (2010, 2016) through LC-MS identified a dominant QTL in IL5-3 that lowered acylsugar levels compared to M82. Additionally, Leckie et al. (2012, 2014) identified QTL on chromosome 5 with overlapping genetic intervals that affected fatty acid profile and also reduced acylsugar level. It was not possible in these studies to determine if the results were due to two linked QTL or due to the same QTL. We introgressed FA5QTL into CU071026 using the line IL5-3, which possesses a 52.6-Mbp chromosome 5 introgression from S. pennellii LA716 (Long et al. 2013). Plants that were homozygous for the majority of the five S. pennellii introgressed regions of CU071026 and also heterozygous for the IL5-3 introgression were selected by MAS from a 380 plant (CU071026 × (IL-5-3 × CU071026)) BC1F1 population. The recombinant BC1F1 plant, 131075-252, was homozygous for S. pennellii introgressions AS2, AS7, and AS10.1 and heterozygous for AS10.2, the top of AS3 from CU071026, and a ca. 3.5 Mbp subregion of the IL5-3 introgression. 131075-252 and plants heterozygous for the entire IL5-3 introgression produced acylsugars with an increase in n-C12 and a decrease in i-C5 fatty acids (data not shown), which is largely consistent with the impact of FA5QTL on acylsugar fatty acid profile as described in Leckie et al. (2014). BC1F1 plants containing the IL5-3 introgression generally accumulated far less acylsugar than plants that lost the introgression, but it was difficult to measure the effect of FA5QTL on acylsugar level since the CU071026 regions, which also affect total acylsugar, were segregating in the population, and so plants differ for presence/homozygosity of those regions. The GC profile of the BC1F1 plants confirms that IL5-3 possesses FA5QTL, that the subintrogression in 131075-252 still contains FA5QTL, and that the QTL functions to alter the fatty acid profile in the presence of the five introgressions of CU071026.
Acylsugar and GC data from selected BC1F1 plants indicated that marker C2_At1g10500, on chromosome 5, from 62,107,578–62,107,796 bp in the 2.5 tomato genomic build (Tomato SL2.50 ITAG2.4), is strongly associated with both decreased acylsugar level and also the change in the fatty acid profile, supporting the idea that either two very closely linked QTL or pleiotropy of one QTL is responsible for both of these traits. It was important to progress to the BC1F2 to obtain lines with further reduction in introgression size, to attempt to separate the QTL for acylsugar level and fatty acid profile, if two linked QTL were controlling these traits.
The purpose of the BC1F2 population was to observe the effect of FA5QTL when homozygous both on acylsugar level and fatty acid profile, which has not previously been reported in an acylsugar-accumulating background, as well as to identify recombinants that could indicate whether the acylsugar level and fatty acid phenotypes were governed by linked QTL or pleiotropy. Seeds from the 131075-252 BC1F1 plant were used to produce a BC1F2 population; this BC1F1 plant was heterozygous for ca. 3.5 Mbp of the original ca. 52.6 Mbp IL5-3 introgression, extending from ca. solcap_snp_sl_29 (60,842,588–60,842,588 bp) to TG23 (63,347,423–63,348,074 bp) (Tomato SL2.50 ITAG2.4 Solgenomics.net), and was also heterozygous for AS10.2 and the top of AS3. Individuals from a 278 plant BC1F2 population were selected by MAS to obtain plants that were homozygous for AS10.2 and top of AS3 and heterozygous or homozygous for presence or absence of the ca. 3.5 Mbp IL5-3 subregion. The acylsugar level of plants homozygous for the 3.5-Mbp IL5-3 subregion is largely the same for plants heterozygous for the sub-IL5-3 introgression, which suggests that FA5QTL is largely dominant in its impacts on total acylsugar level (Fig. 3). Specifically, individuals that had lost the IL5-3 subregion (131245-008, 131245-045, and 131245-072) accumulated acylsugars about the level of CU071026, while plants that were heterozygous (131245-12, 131245-97, and 131245-105) and homozygous (131245-17, 131245-32, 131245-78) for the IL5-3 subregion accumulated acylsugars at ca. 17 and 10 % of CU071026, respectively.
Markers, genotypes, acylsugar level, and selected fatty acid data, diagnostic for the presence of FA5QTL. Plants include CU071026 and selected individuals, representative of a haplotype, out of a BC1F2 population indicating the relative location of FA5QTL at or before snp_29 and up to snp_25859 (solcap_snp_sl_25859). Plant 131245-227 had a recombination such that it lost a small region of AS3 from CU071026 on the top of chromosome 3. Plant 131245-092 was heterozygous for a small region at the top of AS3. i-C5 data Ln(x) transformed and percent CU071026 data cube root transformed prior to analysis to improve normality. Means followed by different letters within a column are significantly different at P < 0.05
The GC-measured fatty acid profiles of the same selected BC1F2 plants differed, depending on the genotype of FA5QTL (Fig. 3). The BC1F2 plants lacking the IL5-3 subregion exhibited an average of 44.4 % i-C5 and 44.1 % n-C12, which is very similar to that of the control CU071026, which averaged 46.8 % i-C5 and 40.2 % n-C12. Selected plants heterozygous for the IL5-3 subregion in the FA5QTL BC1F2 averaged 13.3 % i-C5 and 64.9 % n-C12 fatty acids; this increase in n-C12 and decrease in i-C5 were comparable to that observed in the BC1F1 plants heterozygous for FA5QTL. The fatty acid profile of selections homozygous for the IL5-3 subregion did not fully match that of the heterozygous plants (Fig. 3). Specifically, while the homozygous plants were similar to the heterozygous siblings in having decreased i-C5 fatty acids, they did not show the large increase in n-C12. Six selections homozygous for the IL5-3 subregion averaged 6.5 % i-C5 and 47.7 % n-C12. In addition, in selections homozygous for the IL5-3 subregion, we saw a rise in the proportion of i-C4 and ai-C5 fatty acids (18.6 and 17.4 %), versus selections heterozygous for the IL5-3 subregion which accumulated 8.8 and 7.0 % i-C4 and ai-C5 fatty acids, respectively. Selections that lost the IL5-3 subregion accumulated low levels of i-C4 (3.2 %) and ai-C5 (6.0 %), similar to CU071026, which accumulated 3.7 % i-C4 and 7.3 % ai-C5. These data could indicate that FA5QTL is not fully dominant and or that there is a linked recessive QTL(s) in the IL5-3 subregion that affects the accumulation of i-C4 and or ai-C5 fatty acids. We were unable to identify any recombinant plants in the BC1F2 that appeared to maintain FA5QTL affecting fatty acid profile while losing the negative acylsugar phenotype, which suggests very tight linkage, or more likely, pleiotropy for these two effects.
Utilizing plants recombinant for the IL5-3 introgression and GC data from the BC1F2 plants, we were able to fine map FA5QTL within the original ca. 52.6 Mbp IL5-3 introgression to a region of ca. 1.9 Mbp (Fig. 3). Two selections, 131245-227 and 131245-254, both had low acylsugar levels and fatty acid profiles characteristic of plants homozygous for the IL5-3 subregion. If 131245-227 and 131245-254 are homozygous for FA5, then the putative region is at or before solcap_snp_sl_29 (snp_29) and extending to solcap_snp_sl_25859 (snp_25859) (62,738,979–62,739,013 bp) (Tomato SL2.50 ITAG2.4 Solgenomics.net). Two other recombinant plants, 131245-92 and 269, provide further support for this region. 131245-92 has low levels of acylsugar and a fatty acid profile like that of plants heterozygous for the IL5-3 subregion. 131245-269 has levels of acylsugar and a fatty acid profile like that of CU071026 and selections that lost the subregion. Again this suggests the location of FA5QTL is at or before snp_29 and up to snp_25859. Plant 131245-139 also supports the proposed region for FA5QTL. Plant 131245-139 had low acylsugar level (17.3 % of CU071026), low levels of ai-C5 (4.9 %), and high levels of n-C12 (70.9 %), consistent with the profile of plants heterozygous for the IL5-3 subregion, which implies FA5QTL is before snp_25859. A final recombinant could provide greater delineation. Plant 131245-123 had very low acylsugar level (11.7 % of CU071026), consistent with plants homozygous for the region, but a fatty acid profile more consistent with plants heterozygous for the region. If plant 131245-123 is heterozygous for FA5QTL, it would imply the location is between C2_At1g10500 and snp_25859, but most likely 131245-123 is homozygous for the QTL, which suggests, like the other recombinants, that the location of FA5QTL is at or before snp_29 and up to snp_25859 (ca. 1.9 Mbp).
The dominant QTL identified in Schilmiller et al. (2010) that greatly diminished the acylsugar level of IL5-3 was further elucidated by Schilmiller et al. (2016), where they identified a pair of acylhydrolases on chromosome 5 (Sopen05g030120 and Sopen05g030130) from S. pennellii LA716 and demonstrated that they function in vitro to cleave specific acyl groups from certain acylsucrose molecules. In cultivated tomato, these acylhydrolases are within the IL5-3 full introgression and located at ca. 62.05 Mbp, which is ca. 50,000 bp before C2_At1g10500, and consistent with our FA5QTL mapping interval. Schilmiller et al. (2016) showed that these acylhydrolases greatly contribute to diminished acylsugar levels in the line IL5-3 and provided in vitro evidence that S. pennellii LA716 acylhydrolases preferentially cleave acyl groups from acylsucroses accumulated by cultivated tomatoes, such as M82, rather than acylsucroses from LA716, which accumulates structurally different acylsugars. The results of Leckie et al. (2012) support this idea, since their negative acylsugar level QTL TA5, in the vicinity of FA5QTL, only reduced acylsugar levels to ca. 44 % of haplotypes lacking the FA5QTL region. The lesser degree of reduction in acylsugar level in the Leckie et al. (2012) BC1F1 population could be partly due to FA5QTL being heterozygous, but also, since that population was segregating for most of the S. pennellii LA716 genome, it is likely that some of the SpASAT genes (Schilmiller et al. 2012, 2015; Fan et al. 2016) could have been present, leading to production of acylsucroses that are more like those in LA716 and therefore less subject to acyl cleavage.
The low acylsugar-producing IL5-3 line was also shown to accumulate mostly acylsugars with a long chain fatty acid and only very low amounts of acylsugars with all short acyl chains, such as i-C5 (Schilmiller et al. 2016). This is largely consistent with GC-MS data from the FA5QTL BC1F2 population (Fig. 3), which showed the presence of FA5QTL led to a reduction in i-C5 fatty acids and an increase in n-C12 fatty acids. The effect of FA5QTL in the homozygous condition on fatty acid profile in a higher acylsugar level line, rather than the M82 background, had not previously been reported, and it was intriguing to find different effects on the profile when FA5QTL was homozygous versus heterozygous in the lines bred using CU071026. In particular, the positive effect of FA5QTL, when homozygous, on i-C4 and ai-C5 was unexpected. Leckie et al. (2014) did find a weak association between FA5QTL and ai-C5, moderated by an epistatic interaction with another fatty acid QTL identified in that paper, FA11 but since they were only working in a BC1F1 population, FA5QTL would have been heterozygous, and the effect of FA5QTL in the homozygous condition would not have been seen.
We selected a plant out of the BC1F2 (131245-032) that was homozygous for the ca. 3.5 Mbp IL5-3 subregion and all CU071026 regions and that set seed well in the greenhouse and designated the resulting tomato line FA5/AS, despite its severely reduced level of acylsugar, as the FA5/AS line contains the CU071026 regions necessary for acylsugar production. A depiction of the S. pennellii LA716 introgressions in the FA5/AS line is found in Supplementary Fig. S1. Taken together, these data support the hypothesis of pleiotropy in that the same gene or genes, likely the S. pennellii acylhydrolases, which affect the fatty acid profile, also lead to a reduction in acylsugar level, and it is highly likely that these genes are responsible for the FA5QTL phenotypes. The SpASAT genes are not present in the FA5/AS line, and while these are good candidates for ameliorating the negative acylsugar phenotype of FA5QTL, there presumably are additional genes in S. pennellii that raise acylsugar levels, given that the level of acylsugars produced by CU071026 is only ca. 15 % the level of the wild species (Leckie et al. 2012). Whether some or all of the SpASAT genes, or other classes of acylsugar level QTL, are sufficient to recover the CU071026 level of acylsugar in lines that also possess FA5QTL remains to be seen, but until the necessary regions from S. pennellii LA716 are identified and combined with FA5QTL, it is unlikely the current FA5/AS line containing FA5QTL can be utilized against insects due to very low acylsugar accumulation.
Simultaneous characterization of all modified fatty acid lines
Previous studies investigating the broad sense heritability of secondary metabolites in tomato, including some acylsugar compounds, suggest that heritability of secondary metabolites in tomato is often quite high (Alseekh et al. 2015). In agreement with that study, total acylsugar level displays high broad sense heritability in our acylsugar lines (0.76) (Supplementary Table S3). Despite high heritability, acylsugar-accumulating tomatoes produce different quantities of acylsugar in different environments (Shapiro et al. 1994), indicating that the amount of acylsugar accumulated exhibits significant genotype by environment interaction. This variability makes it difficult to compare the new modified fatty acid lines for acylsugar-related traits using data collected at different times and in different greenhouses as the lines were developed. Similarly, it was possible that environmental factors could impact fatty acid profiles of the acylsugars produced. Therefore, a final experiment growing all modified fatty acid acylsugar lines and controls in a replicated trial under greenhouse conditions was necessary to simultaneously characterize the lines for acylsugar level, density of glandular trichomes, and their acylsugar profile, both for fatty acid acyl group through GC-MS, and type of acylsugar through LC-MS.
Acylsugar level and trichome density
The fatty acid QTL modified the fatty acids of the acylsugars produced as expected but also impacted the acylsugar levels of several of the resulting modified fatty acid acylsugar lines. The FA2/AS and FA7/AS lines accumulated higher (119.2 and 121.9 %, respectively) acylsugar levels than CU071026 (Table 2). It is possible that the introgressions containing FA2QTL and FA7QTL introgressions also contain some minor QTL from S. pennellii LA716 affecting acylsugar level or that the QTL responsible in the introgressions for alteration of fatty acid profile also impact acylsugar level. If there are QTL for increased acylsugar level in the FA2/AS and FA7/AS lines, the effect of these QTL might be influenced by the environment, since past characterization of these lines in field and greenhouse settings (data not shown) indicated that their acylsugar levels are not always significantly different from those of CU071026. The FA8/AS line appeared to have a slightly lower acylsugar level (81.1 %) than the CU071026 control, although the difference was not statistically significant. The acylsugar level of the FA5/AS line was strongly reduced, averaging only 16.2 % the level of CU071026 (Table 2), indicating that the regions from S. pennellii LA716 ameliorating this impact of FA5QTL on acylsugar level must be identified and introgressed to obtain a FA5/AS tomato line that produces high acylsugar levels.
Because prior work showed that acylsugar level could be increased with or without an increase in trichome density (Leckie et al. 2012), we evaluated the density of glandular trichomes among the modified fatty acid lines and their controls. This characterization allows differentiation between increased trichome density and increased acylsugar production per trichome as mechanisms for overall increased acylsugar levels. The density of type IV trichomes varied among the lines tested, with increased trichome density positively correlated with increased acylsugar level (r = 0.66). The type IV trichome density of CU071026 and the FA2/AS and FA7/AS lines was all equivalent; however, the type IV trichome density of the FA5/AS line was lower and the density of type IV trichomes in the FA8/AS line was marginally lower than the other three entries (Table 2).
Type IV glandular trichome types have repeatedly been implicated as the predominant trichome type for acylsugar production (Fobes et al. 1985; Goffreda et al. 1990; Slocombe et al. 2008). In particular, the SpASAT1-4 genes, which are integral in attachment of acyl groups to the sugar backbone in acylsugar biosynthesis have been shown to be expressed in type IV trichomes (Schilmiller et al. 2012, 2015; Fan et al. 2016). While density of type IV trichomes was positively correlated with acylsugar level when comparing the fatty acid lines, the density of type IV trichomes alone does not account for differences in acylsugar levels among lines. Type VI glandular trichome density also varied between entries but was not positively correlated with acylsugar accumulation (r = −0.16) as expected based on the role of type VI trichomes in production of other specialized metabolites, such as terpenes (Coates et al. 1988; Frelichowski and Juvik 2001; Li et al. 2004). These results imply that differences in acylsugar levels among lines could be influenced by type IV trichome density but are more likely due to variation in acylsugar biosynthesis rather than trichome density.
Acylsugar level can also be influenced by QTL that increase or decrease the density of the trichomes that produce and exude these compounds. Comparison of acylsugar-producing sister lines with and without the chromosome 6 S. pennellii LA716 QTL, named TA6, showed that its presence increased both the total acylsugar level and the density of type IV trichomes that exude acylsugar droplets (Leckie et al. 2012). While the modified fatty acid lines have variation for acylsugar level and trichome density, none of the fatty acid lines possess the TA 6 acylsugar level QTL or the TA 10.1 acylsugar level QTL that increases acylsugar level without affecting trichome density (Leckie et al. 2012). The acylsugar level and trichome data from the fatty acid lines suggest type IV trichome density is an important morphological feature for acylsugar level in some of the modified fatty acid lines but that modulation of acylsugar level through biosynthesis could also be a major component contributing to the differential acylsugar levels observed. It is possible that adding one or both of the acylsugar level QTL (TA 6 or TA 10.1) to the fatty acid lines could further improve the acylsugar level/type characteristics of the resulting lines.
Fatty acid characterization from GC-MS
The results from the GC-MS analysis of CU071026 versus the new modified fatty acid lines closely matched predictions based on prior QTL analyses (Leckie et al. 2014). Consistent with previous characterizations, the acylsugars of the FA2/AS line included the extended branch chain fatty acids i-C11 and i-C13 at 8.7 and 3.2 % respectably, whereas in the acylsugars of CU071026, these fatty acids were either found at trace levels or not detected (Table 2). The acylsugars of the FA2/AS line also possessed reduced levels of n-C12 and slightly increased levels of i-C5 compared to the acylsugars of the CU071026 control. The fatty acid profile of the acylsugars of the FA7/AS line was also consistent with previous characterization of lines during their development; the acylsugars of this line have an increase in n-C10 fatty acids (11.3 %) over acylsugars of CU071026 (1.5 %) and a reduction in the level of i-C5 (37.9 %) compared to that of CU071026 (48.1 %) (Table 2). The FA8/AS line data were also generally consistent in profile with previous characterization of lines during their development; the acylsugars of the FA8/AS line show increased i-C4 fatty acids (12.5 %) over the acylsugars of CU071026 (3.4 %) as well as decreased i-C5 fatty acids (16.1 %) versus that in CU071026 (48.1 %) (Table 2). Additionally, FA8/AS appears to accumulate increased n-C12 fatty acids, which was not previously observed. The FA5/AS line GC-MS data deviated from previous characterization in that the FA5/AS line in the replicated trial accumulated higher levels of i-C4 (40.5 %) and very low levels of ai-C5 (3.5 %) and i-C5 (9.4 %), than was seen in initial characterization of BC1F2 selections homozygous for FA5QTL subregion. This difference could be due in part to the difficulties inherent in characterizing fatty acids present at low levels by GC-MS. To better understand the effect of FA5QTL on acylsugar chemistry, it is necessary to identify and combine any necessary epistatic QTL from S. pennellii LA716 that will allow recovery of higher levels of acylsugar in the presence of FA5QTL. With the exception of the FA5/AS line, all modified acylsugar lines displayed remarkable consistency in fatty acid profile at different times and in different environments, suggesting fatty acid profile is minimally impacted by the environment. Broad sense heritability estimates were high for all the major fatty acids accumulated by the acylsugars lines from 2014 and 2015 and are displayed in Supplementary Table S3.
Acylsugar characterization from LC-MS
To further characterize the fatty acid lines, LC-MS data was collected to provide information about not only the fatty acids accumulated by each line but also to provide greater detail on the major acylsugars produced, including the number and length of fatty acids and to which sugar they are esterified (Fig. 4). Representative LC-MS chromatograms for each fatty acid line can be found in online Supplementary Fig. S2. LC-MS is a sensitive technique that can readily detect the low levels of acylsugars produced by cultivated tomato. Although the LC-MS procedure cannot differentiate between orientations in fatty acids of the same length, such as ai-C5 and i-C5, GC-MS can differentiate between these, and therefore the joint analysis using both GC-MS and LC-MS data provides greater detail regarding the acylsugar profiles of each fatty acid line.
Hierarchical cluster analysis, with Pearson correlation using a pairwise average-linkage clustering method, indicating the predominant acylsugars accumulated by each fatty acid line. Three samples for each genotype were analyzed. Color across a row indicates relative levels of the respective acylsugar, with red indicating samples with the highest levels detected and blue/purple indicating low or no detection relative to the highest sample. Superscript a: The mass-to-charge ratio for each acylsugar followed by retention time in minutes. Superscript b: Acylsugar nomenclature indicates S for sucrose backbone of the molecule, as well as the number of fatty acid acyl chains (two to four) with their cumulative length in carbons that are esterified to the sugar followed by the lengths in number of carbons of each acyl group in the respective acylsugar. Superscipt c: Proposed acylgroup number and length for acylsugar; identification hampered by low abundance and peak overlap
CU071026 predominantly accumulates three major acylsugars: S4:17 (ID 53), S3:22 (ID 56), and S4:24 (ID 55) (Fig. 4). These acylsugars support the GC-MS fatty acid data, as all three acylsugars contain C5 and C12 fatty acids, which are the principal fatty acid chain lengths in CU071026. The FA2/AS line accumulates the three major acylsugars found in CU071026 but additionally possesses significant amounts of two acylsugars that are much lower in CU071026: S3:21 (ID 33) and S4:25 (ID 30). These two acylsugars contain C11 and C13 fatty acids, respectively, which accumulate in the FA2/AS line but are at trace or undetectable amounts in CU071026 (Supplementary Fig. S3). The FA7/AS line also accumulates the three major acylsugar peaks found in CU071026 and also has greatly increased relative abundance of two acylsugars accumulated at very low levels in CU071026, S3:20 (ID 45) and S4:22 (ID 46) (Supplementary Fig. S4). Additionally, the FA7/AS line accumulates low levels of several other acylsugars that show very low abundance in CU071026, including S3:19 (ID 42) and S4:21 (ID 43). The increase in abundance of C10 containing acylsugars for the FA7/AS line shown by LC-MS is consistent with GC-MS data which indicates n-C10 fatty acids are more prevalent in this line. The FA8/AS line accumulates the three major acylsugars in CU071026 but also produces significant quantities of a number of acylsugars not significantly accumulated by CU071026, including S4:15 (ID 18), S3:21 (ID 24), and S4:23 (ID 23). These acylsugars all contain C4 fatty acids (Supplementary Fig. S5), and some contain C12 fatty acids which are consistent with FA8/AS line GC-MS data indicating an increase in levels of i-C4 and n-C12 fatty acids. There were also two acylglucose isomers detected at low abundance in the FA8/AS line (IDs 4 and 5) that were not detected in the other fatty acid lines. As all the fatty acid lines are lacking several important QTL necessary for production of acylglucoses (Smeda et al. unpublished), the detection of acylglucoses in the FA8/AS line was unexpected and suggests FA8QTL or a linked QTL within the IL8-1-1 introgression could be involved in acylglucose biosynthesis. The FA5/AS line was found to accumulate similar types of acylsugars to CU071026; however, in contrast to data from the GC-MS based fatty acid characterization of the FA5/AS with all lines together, LC-MS data showed the FA5/AS line accumulated an increased proportion of acylsugars containing a long chain fatty acid, and reduced quantities of acylsugars with all short chain acyl groups, consistent with the results in Schilmiller et al. (2016). It is possible that fatty acid levels near the detection limit in GC-MS analysis will result in higher variability between repeated samples and suggest the profile of tomatoes that accumulate low levels of acylsugar, such as the FA5/AS line, should be characterized through LC-MS for consistent results.
For several acylsugars identified in the LC-MS analysis, there were clear chromatographic separations of compounds having identical mass and indistinguishable mass spectra, for example S3:20 (5,5,10) (ID 45) with a retention time of 8.22 min and S3:20 (5,5,10) (ID 28) with a retention time of 8.07 min (Fig. 4). These acylsugar isomers likely differ in either the position of acyl chain attachment or in the branching of the acyl chains. There are several instances of fatty acid QTL influencing the relative abundance of different acylsugar isomers. For example, each line accumulates some level of S3:20 (5,5,10) (ID 45) (Supplementary Table S4); however, the FA2/AS line also accumulates a significant amount of S3:20 (5,5,10) (ID 28) that is not detected in CU071026 or the FA7/AS line and is seen at only very low levels in the FA5/AS and FA8/AS lines. The difference between these two isomers would require purification and nuclear magnetic resonance (NMR) analysis for clarification and could be pursued in future work.
The hierarchical clustering analysis (HCA) in Fig. 4 separates the fatty acid lines into several clades. Raw chromatogram peak area data used to generate the HCA can be found in online Supplementary Table S4. As expected, the presence of FA8QTL largely separates the fatty acid lines into two main clusters. Acylsugars with IDs 6 to 25 commonly contain C4 fatty acids and are significantly accumulated in the FA8/AS line and largely absent in the other acylsugar lines, which is due to the effect of FA8QTL. Acylsugars with IDs 26 to 56, conversely, are generally accumulated in CU071026, and the FA2/AS and FA7/AS lines, and largely absent in the FA8/AS line. Further elucidation of the impact of fatty acid QTL on the fatty acid combinations within acylsugars and the locations of fatty acid attachment could allow greater understanding of acylsugar biosynthesis, how acylsugars interact with insects, and selection of optimal acylsugar lines for insect resistance.
The effect of the fatty acid QTL on acylsugar diversity
A broad question that can be addressed by looking at the combination of GC-MS and LC-MS data on the acylsugar lines is whether addition of the fatty acid QTL into CU071026 increases the diversity of fatty acid acyl groups and or the diversity of acylsugars accumulated. It is possible that when a novel acylsugar is accumulated in a line possessing an additional QTL, the acylsugar replaces acylsugar(s) accumulated in lines lacking the QTL, so that the addition of fatty acid QTL might lead to accumulation of acylsugars not seen in CU071026 but that this gain could be coupled with the loss of acylsugars produced in CU071026. Alternately, addition of a novel acylsugar not accumulated in CU071026 could be additive rather than a replacement of a previously accumulated acylsugar. GC-MS data indicates there are five fatty acid acyl groups (i-C4, ai-C5, i-C5, n-C10, and n-C12) that are significantly accumulated by all the fatty acid lines and CU071026 (Table 2). The FA5/AS, FA7/AS, and FA8/AS lines vary in the relative proportions of these five fatty acids but do not accumulate detectable levels of novel fatty acids not found in CU071026. Conversely, the FA2/AS line accumulates the five fatty acids in CU071026 as well as detectable levels of two fatty acids (i-C11 and i-C13) not abundant in CU071026. These data indicate that addition of FA2QTL increases acyl chain diversity compared to CU071026, but the QTL in lines FA5QTL, FA7QTL, and FA8QTL do not increase acyl chain diversity.
Concerning LC-MS data, 56 acylsugars are identifiable among the fatty acid lines and CU071026 (Fig. 4). We can evaluate the effect of introgression of the fatty acid QTL on acylsugar diversity by comparing the lines for acylsugars that are accumulated at moderate to high levels in respective lines. The acylsugars that are the darkest blue color in Fig. 4 are accumulated at low levels or are not detectable in the respective line. If at least two of the three samples per genotype are above the background dark blue, that acylsugar is considered present in that line. Using this metric, CU071026 accumulates 23 of the 56 identifiable acylsugars. Not surprising, the FA2/AS line accumulated an increased number of acylsugars (31), some of which contained C11 and C13 acyl groups, likely the novel i-C11 and i-C13 fatty acid acyl groups detected in GC-MS analysis. LC-MS analysis revealed the FA7/AS and FA8/AS lines also accumulated an increased diversity of acylsugars over CU071026 (30 each), comparable to the FA2/AS line. Unlike FA2QTL, which partly seems to increase acylsugar diversity by incorporating novel acyl groups, FA7QTL and FA8QTL seem to increase diversity by modulating increased or decreased incorporation of existing upregulated or downregulated fatty acid acyl groups. The FA5/AS line displayed a lower diversity of acylsugars due to the low levels of acylsugar in this line, likely resulting from the presence of the acylhydrolases discussed in Schilmiller et al. (2016). Taken together, the LC-MS data indicate that addition of FA2QTL, FA7QTL and FA8QTL each leads to an increased diversity of acylsugars accumulated.
The additional acylsugars in the FA7/AS line did not result in a substantial tradeoff since all but one of the acylsugars moderately accumulated by CU071026 were still accumulated by the FA7/AS line (Fig. 4). In the FA2/AS line, the addition of eight acylsugars resulted in a slight tradeoff; four acylsugars accumulated by CU071026 were no longer moderately accumulated in the FA2/AS line. The FA8/AS line accumulated seven additional acylsugars, but this resulted in a strong tradeoff (Fig. 4), with only six acylsugars in common between CU071026 and the FA8/AS line.
Perspective to the diversity metrics discussed in this study can be gained by comparison with recent published work concerning acylsugar biosynthesis and chemistry (Schilmiller et al. 2010, 2015; Fan et al. 2016). It is evident that there is, at most, one long (C9 to C13) chain acyl group found in each of the 56 identifiable acylsugars accumulated by the fatty acid lines and CU071026 (Fig. 4); the long chain acyl group is almost exclusively found in one location in tomato acylsucroses, whereas there are up to four positions at which short chain (C4, C5, C6) acyl groups are commonly found in tomatoes (Schilmiller et al. 2015; Fan et al. 2016). With this in mind, the added diversity in acylsugars provided by the addition of FA2QTL and FA7QTL has limited impact in diversifying the acylsugars produced since these two QTL mostly alter the long chain fatty acids. Despite the addition of novel fatty acids, the FA2/AS line only accumulates moderate levels of one additional acylsugar compared with the FA7/AS line because there are limited long chain acyl group attachment sites open to diversification. There is not much tradeoff in acylsugar diversity produced in the FA2/AS and FA7/AS lines compared to CU071026, since these three lines have similar levels of ai-C5 and i-C5 fatty acids, and these short chain acyl groups are ubiquitous in almost all acylsugars accumulated by these lines. On the other hand, FA8QTL increases the diversity of short chain fatty acids, which leads to increased diversity of acylsugar chemotypes accumulated in the FA8/AS line, but the loss of some of the acylsugar chemotypes found in CU071026. The FA8/AS line has increased levels of i-C4, which is only found at low levels in CU071026, and greatly reduced levels of i-C5, which is the most common short chain acyl group in CU071026. Furthermore, since short chain acyl groups are part of every acylsugar identified in the fatty acid lines and CU071026 (Fig. 4), there are few acylsugars in the FA8/AS line that do not incorporate at least one i-C4 acyl group. In fact, many of the acylsugars no longer accumulated at substantial levels in the FA8/AS line contain at least two C5 acyl groups. This replacement of at least one i-C5 group with one i-C4 acyl group is most likely responsible for the limited overlap of acylsugars between CU071026 and the FA8/AS line. This idea is supported by LC-MS data from Schilmiller et al. (2010) showing i-C5 replacement by i-C4 acyl groups in the line IL8-1-1 versus M82. Additionally, Ning et al. (2015) showed that a feedback insensitive isopropylmalate synthase on chromosome 8 governs an increase in C4 and a decrease in C5 acyl groups in cultivated and wild tomato.
Conclusions
An important question when transferring the FA5, FA7, and FA8QTL was whether the QTL have the impact on acylsugar accumulation in the resulting line that was predicted based on prior QTL analysis. As noted by Bernardo (2008) regarding work in maize, the majority of QTL identified never find their way into released varieties. The common explanation for this observation across crops is that the QTL do not have the expected impact after transfer from the background in which they were discovered, possibly due to missing epistatic interactions. When dealing with qualitative traits governed by one or two QTL, the likelihood of successful transfer and expected function is greater than when dealing with quantitative traits. Since there are many QTL that affect both acylsugar level and/or chemistry, it is difficult to predict how a QTL will behave in a different genetic background. For example, pertinent to FA5QTL, the recently discovered acylhydrolases (Schilmiller et al. 2016) that cleave particular acyl groups from specific locations on acylsucroses depend on the ASAT genes, which is likely why the acylsucroses of S. pennellii LA716 are less subject to acyl group cleavage than the acylsucroses of tomato, such as in the FA5/AS line. Therefore, it is imperative to understand acylsugar biochemistry to successfully utilize QTL that impact acylsugar chemistry because of the potential for epistatic interactions.
Analysis of the new tomato lines bred by transfer of FA2QTL, FA5QTL, and FA7QTL showed alteration in acylsugar profiles that largely matched the expectations for these QTL based on QTL analysis. The existence of epistatic interactions among acylsugar QTL affecting acylsugar level, sugar component of acylsugars, and fatty acid components of acylsugars was already demonstrated in QTL mapping populations (Leckie et al. 2012, 2013, 2014) and will be a factor in development of lines with desired acylsugar levels and chemotypes. Production of lines combining two or more of the fatty acid QTL would be needed to test whether similar epistatic relationships are observed in the resulting lines.
The incorporation of FA2QTL, FA7QTL. and FA8QTL into CU071026, creating the lines FA2/AS, FA7/AS, and FA8/AS, respectively, led to an increase in the diversity of either fatty acid acyl groups or acylsugars accumulated in these lines. A logical question to ask is whether the functionality of the acylsugars is altered by the increased diversity. From the results of Leckie et al. (2016), it is evident that acylsugar chemistry can have an impact on the efficacy of insect deterrence. It is possible that production of particular acyl groups, or modulation of the proportions of particular acyl groups, played a role in the differential insect control observed in that study. The results of Leckie et al. (2016) also demonstrate that synergy is an important element in the functionality of acylsugars as a defense, which suggests that an increased diversity of acyl groups and acylsugars could lead to greater opportunity for synergy and therefore improved insect resistance. Whether the fatty acid lines differ for control of various insects and the role of acyl group and acylsugar diversity in mediating the efficacy of insect resistance will be evaluated in future studies.
The tomato lines completed to date, and additional lines nearing completion, are being developed to serve as a research platform with broad utility. These lines could be used (1) for research directed at further elucidating acylsugar biosynthesis and its regulation, such as the genes controlling FA2QTL and FA7QTL, which have not yet been elucidated (2) for a range of entomological research including efficacy of acylsugar-mediated control of diverse tomato insect and arthropod pest species by the different lines, identification of acylsugar levels and chemotypes with the optimal impact on each insect species, and study of the mechanism by which acylsugar-mediated insect resistance operates against insect and arthropod pest species (3) to be utilized as breeding material for the transfer of acylsugar QTL to tomato lines with optimal acylsugar profiles for control of targeted insect and arthropod pest species with reduction/elimination of pesticides. As each line is completed, characterized, and its seed is sufficiently increased, the line will be provided, under MTA, upon request.
Abbreviations
- ai-C5:
-
2-Methylbutanoate (anteiso branched 5 carbon acyl group)
- i-C4:
-
2-Methylpropanoate (iso branched 4 carbon acyl group)
- i-C5:
-
3-Methylbutanoate (iso branched 5 carbon acyl group)
- i-C10:
-
8-Methylnonanoate (iso branched 10 carbon acyl group)
- i-C11:
-
9-Methyldecanoate (iso branched 11 carbon acyl group)
- i-C13:
-
11-Methyldodecanoate (iso branched 13 carbon acyl group)
- n-C10:
-
n-Decanoate (straight chain 10 carbon acyl group)
- n-C12:
-
n-Dodecanoate (straight chain 12 carbon acyl group)
References
Alseekh S, Zamir D et al (2015) Identification and mode of inheritance of quantitative trait loci for secondary metabolite abundance in tomato. Plant Cell 27:485–512. doi:10.1105/tpc.114.132266
Bates D, Maechler M, Bolker B, Walker S (2014) Linear mixed-effects models using Eigen and S4. R package version 1.0-5
Bernardo R (2008) Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci 48:1649–1664. doi:10.2135/cropsci2008.03.0131
Blauth SL, Churchill GA, Mutschler MA (1998) Identification of quantitative trait loci associated with acylsugar accumulation using intraspecific populations of the wild tomato, Lycopersicon pennellii. Theor Appl Genet 96:458–467. doi:10.1007/s001220050762
Blauth SL, Steffens JC, Churchill GA, Mutschler MA (1999) QTL analysis of acylsugar fatty acid constituents using intraspecific populations of the wild tomato Lycopersicon pennellii. Theor Appl Genet 99:373–381. doi:10.1007/s001220051247
Burke B, Goldsby G, Mudd JB (1987) Polar epicuticular lipids of Lycopersicon pennellii. Phytochemistry 26:2567–2571. doi:10.1016/S0031-9422(00)83879-0
Coates RM, Denissen JF, Juvik JA, Babka BA (1988) Identification of alpha-santalenoic and endo-beta-bergamotenoic acids as moth oviposition stimulants from wild tomato leaves. J Org Chem 53:2186–2192. doi:10.1021/jo00245a012
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847. doi:10.1038/35081178
Eshed Y, Zamir D (1994) A genomic library of Lycopersicon pennellii in L. esculentum: a tool for fine mapping of genes. Euphytica 79:175–179. doi:10.1007/BF00022516
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
Fan P, Last RL et al (2016) In vitro reconstruction and analysis of evolutionary variation of the tomato acylsucrose metabolic network. Proc Natl Acad Sci 113:E239–E248. doi:10.1073/pnas.1517930113
Fancelli M, Vendramim JD, Frighetto RTS, Lourencao AL (2005) Glandular exudate of tomato genotypes and development of B. tabaci (Genn.) (Sternorryncha: Aleyrodidae) biotype B. Neotrop Entomol 34:659–665. doi:10.1590/S1519-566X2005000400017
Fobes JF, Mudd J, Marsden M (1985) Epicuticular lipid on the leaves of L. pennellii and L. esculentum. Plant Physiol 77:567–570. doi:10.1007/s11032-013-9849-5
Frelichowski JE, Juvik JA (2001) Sesquiterpene carboxylic acids from a wild tomato species affect larval feeding behavior and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J Econ Entomol 94:1249–1259. doi:10.1603/0022-0493-94.5.1249
Ghosh B, Westbrook TC, Jones AD (2014) Comparative structural profiling of trichome specialized metabolites in tomato (Solanum lycopersicum) and S. habrochaites: acylsugar profiles revealed by UHPLC/MS and NMR. Metabolomics 10:496–507. doi:10.1007/s11306-013-0585-y
Goffreda JC, Mutschler MA (1989) Inheritance of potato aphid resistance in hybrids between Lycopersicon esculentum and L. pennellii. Theor Appl Genet 78:210–216. doi:10.1007/BF00288801
Goffreda JC, Mutschler MA, Steffens JC (1990) Association of epicuticular sugars with aphid resistance in hybrids with wild tomato. J Am Soc Hortic Sci 117:161–164
Hawthorne DM, Shapiro JA, Tingey WM, Mutschler MA (1992) Trichome-borne and artificially applied acylsugars of wild tomato deter feeding and oviposition of the leaf- miner, Liriomyza trifolii. Entomol Exp Appl 65:65–73. doi:10.1111/j.1570-7458.1992.tb01628.x
Holland JB, Nyquist WE, Cervantes-Martínez CT (2003) Estimating and interpreting heritability for plant breeding: an update. Plant breeding reviews 22:9–112
JMP®, Version 11. SAS Institute Inc., Cary, NC, 1989-2007
Juvik J, Shapiro JA, Young TE, Mutschler MA (1994) Acyl-glucoses of the wild tomato Lycopersicon pennellii alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua. J Econ Entomol 87:482–492. doi:10.1007/s11032-013-9849-5
Kim J, Kang K, Gonzales-Vigil E, Shi F, Jones D, Barry CS, Last RL (2012) Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the acyltransferase2 locus in the wild tomato Solanum habrochaites. Plant Physiol 160:1854–1870. doi:10.1104/pp.112.204735
King R, Calhoun A (1988) 2,3-di-O_ and 1,2,3-tri-O-Acylated glucose esters from the glandular trichomes of Datura metel. Phytochemistry 27:3761–3763. doi:10.1016/0031-9422(88)83013-9
King RR, Calhoun LA, Singh RP (1988) 3,4-di-O- and 2,3,4-tri-O-Acylated glucose esters from the glandular trichomes of Nontuberous solanum species. Phytochemistry 27:3765–3768. doi:10.1016/0031-9422(88)83014-0
King RR, Pelletier Y, Singh RP, Calhoun LA (1986) 3,4-di-O-Isobutyryl-6-O-caprylsucrose: the major component of a novel sucrose ester complex from the type B glandular trichomes of Solanum berthaultii hawkes (Pl 473340). J Chem Soc Chem Comm 14:1078–1079. doi:10.1039/C39860001078
Leckie BM, Mutschler MA et al (2016) Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS One 11:1–19. doi:10.1371/journal.pone.0153345
Leckie BM, Halitschke R, De Jong DM, Smeda JR, Kessler A, Mutschler MA (2014) Quantitative trait loci regulating the fatty acid profile of acylsugars in tomato. Mol Breed 34:1201–1213. doi:10.1007/s11032-013-9849-5
Leckie BM, DeJong DM, Mutschler MA (2012) Quantitative trait loci increasing acylsugars in tomato breeding lines and their impacts on silverleaf whiteflies. Mol Breed 31:957–970. doi:10.1007/s11032-012-9746-3
Leckie BM, DeJong DM, Mutschler MA (2013) Quantitative trait loci regulating sugar moiety of acylsugars in tomato. Mol Breed 31:957–970. doi:10.1007/s11032-013-9849-5
Li L, Howe GA et al (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16:126–143. doi:10.1105/tpc.017954
Liedl BE, Lawson DM, White KK, Shapiro JA, Cohen DE, Carson WG, Trumble JT, Mutschler MA (1995) Acylglucoses of the wild tomato Lycopersicon pennellii alters settling and reduces oviposition of Bemisia argentifolii. J Econ Entomol 88:742–748. doi:10.1093/jee/88.3.742
Long W, Li Y, Zhou W, Ling HQ, Zheng S (2013) Sequence-based SSR marker development and their application in defining the introgressions of LA0716 (Solanum pennellii) in the background of cv. M82 (Solanum lycopersicum). PLoS One 8:e81091. doi:10.1371/journal.pone.0081091
Mutschler MA, Wintermantel WM (2006) Reducing virus associated crop loss through resistance to insect vectors. In: Loebenstein G, Carr JP (eds) Natural resistance mechanisms of plants to viruses. Springer, Dordrecht, pp. 241–260
Ning J, Last RL et al (2015) A feedback insensitive isopropylmalate synthase affects acylsugar composition in cultivated and wild tomato. Plant Physiol 169:1821–1835. doi:10.1104/pp.15.00474
Ohya I, Shinozaki Y, Tobita T, Takahashi H, Matsuzaki T (1996) Sucrose esters from the surface lipids of Petunia hybrida. Phytochemistry 41:787–789. doi:10.1016/0031-9422(95)00679-6
Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP (2006) GenePattern 2.0. Nat Genet 38:500–501
Rodriguez AE, Tingey WM, Mutschler MA (1993) Acylsugars produced by type IV trichomes of Lycopersicon pennellii (Corr.)D’Arcy deter settling of the green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). J Econ Entomol 86:34–39. doi:10.1093/jee/86.1.34
Schilmiller AL, Charbonneau AL, Last RL (2012) Identification of a BAHD acetyltransferase that produces protective acyl sugars in tomato trichomes. Proc Natl Acad Sci U S A 109:16377–16382. doi:10.1073/pnas.1207906109
Schilmiller AL, Gilgallon K, Ghosh B, Jones AD, Last RL (2016) Acylsugar acylhydrolases: carboxylesterase catalyzed hydrolysis of acylsugars in tomato trichomes. Plant Physiol 170:1331–1344. doi:10.1104/pp.15.01348
Schilmiller AL, Last RL, Pichersky E (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J 54:702–711. doi:10.1111/j.1365-313X.2008.03432.x
Schilmiller AL, Moghe GD, Fan P, Ghosh B, Ning J, Jones AD, Last RL (2015) Functionally divergent alleles and duplicated loci encoding an acyltransferase contribute to acylsugar metabolite diversity in Solanum trichomes. Plant Cell 27:1002–1017. doi:10.1105/tpc.15.00087
Schilmiller AL, Shi F, Kim J, Charbonneau A, Holmes D, Jones AD, Last RL (2010) Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 62:391–403
Setter TL, Flannigan BA, Melkonian J (2001) Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci 41:1530–1540. doi:10.2135/cropsci2001.4151530x
Severson RF, Johnson AW, Jackson DM (1985) Cuticular constituents of tobacco: factors affecting their production and their role in insect and disease resistance and smoke quality. Recent Adv Tobacco Sci 11:105–174
Shapiro J, Steffens J, Mutschler MA (1994) Acylsugars of the wild tomato Lycopersicon pennellii in relation to its geo-graphic distribution. Biochem Syst Ecol 22:545–561. doi:10.1016/0305-1978(94)90067-1
Sim SC, Durstewitz G, Plieske J, Wieseke R, Ganal MW, Van Deynze A et al (2012) Development of a large SNP genotyping array and generation of high-density genetic maps in tomato. PLoS One 7:e40563. doi:10.1371/journal.pone.0040563
Slocombe SP, Schauvinhold I, McQuinn RP, Besser K, Welsby NA, Harper A, Aziz N, Li Y, Larson TR, Giovannoni J, Dixon RA, Broun P (2008) Transcriptomic and reverse genetic analyses of branched-chain fatty acid and acyl sugar production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol 148:1830–1846. doi:10.1104/pp.108.129510
Weinhold A, Baldwin IT (2011) Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. PNAS 108:7855–7859. doi:10.1073/pnas.1101306108
Wink M (2010) Functions and biotechnology of plant secondary metabolites. Wiley-Blackwell, Oxford
Acknowledgements
We thank Andre Kessler for valuable discussions on the implications of metabolite diversity as it relates to acylsugar-mediated insect control and critical reading of the manuscript.
We also thank Darlene DeJong for critical assistance and guidance with running molecular markers and acylsugar assays.
This project was supported in part by Agriculture and Food Research Initiative Competitive Grant no. 2013-67013-21135 from the USDA National Institute of Food and Agriculture, by the USDA National Institute of Food and Agriculture, Hatch project NYC-149440 (to M.A.M), and by National Science Foundation grant IOS–1025636 (to R.L.L.). Smeda was supported in part by an NSF GRFP graduate fellowship, as well as for one semester on by Agriculture and Food Research Initiative Competitive Grant no. 2010-85117-20551.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Smeda, J.R., Schilmiller, A.L., Last, R.L. et al. Introgression of acylsugar chemistry QTL modifies the composition and structure of acylsugars produced by high-accumulating tomato lines. Mol Breeding 36, 160 (2016). https://doi.org/10.1007/s11032-016-0584-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0584-6