Abstract
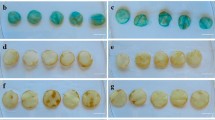
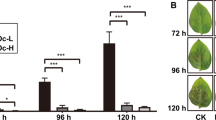
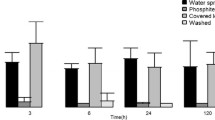
Land plants synthesize phenolic compounds involved in plant defense against invading pathogens through the phenylpropanoid pathway. Although not considered as part of the phenylpropanoid pathway, plant polyphenol oxidases (PPOs) are enzymes that catalyze cresolase and catecholase reactions on several phenolic compounds. Here, transgenic potato (Solanum tuberosum) tubers with downregulated PPO genes (-PPO) were challenged with the oomycete pathogen Phytophthora infestans to investigate the interactions between PPO, phenylpropanoid metabolism, and disease resistance. We found that pathogen invasiveness was reduced in -PPO lines, while microscopic evidences suggested that the mechanism underlying the defense response involved the participation of phenolic compounds. Detailed metabolite-profiling analyses demonstrated that the concentration of metabolites related to the phenylpropanoid pathway and chlorogenate in particular was largely altered in PPO-downregulated tubers. Silencing of PPO caused a shift in metabolism from phenylpropanoid precursors to downstream phenylpropanoid products. The presented results suggest that downregulation of PPO redirects the phenylpropanoid metabolism leading to the accumulation of defensive phenolic compounds in the plant cells, consequently enhancing resistance to the pathogen. These results emphasize the importance of components acting in parallel to canonical metabolic pathway constituents in influencing plant metabolism and reveal new scenarios for modulating the levels of phenolics in crops.





Similar content being viewed by others
References
Ah-Fong AM, Bormann-Chung CA, Judelson HS (2008) Optimization of transgene-mediated silencing in Phytophthora infestans and its association with small-interfering RNAs. Fungal Genet Biol 45:1197–1205
Andreu AB, Guevara MG, Wolski EA, Daleo GR, Caldiz DO (2006) Enhancement of natural disease resistance in potatoes by chemicals. Pest Manag Sci 62:162–170
Araji S et al (2014) Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol 164:1191–1203
Balakumar T, Gayathri B, Anbudurai PR (1997) Oxidative stress injury in tomato plants induced by supplemental UV-B radiation. Biol Plant 39:215–221
Beerhues L, Kombrink E (1994) Primary structure and expression of mRNAs encoding basic chitinase and 1,3-beta-glucanase in potato. Plant Mol Biol 24:353–367
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Ben Abdullah F (2009) Saline water irrigation effects on antioxidant defense system and proline accumulation in leaves and roots of field-grown olive. J Agric Food Chem 57:11484–11490
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Ben Abdullah F (2010) Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agric Food Chem 58:4216–4222
Bennett M, Gallagher M, Fagg J, Bestwick C, Paul T, Beale M, Mansfield J (1996) The hypersensitive reaction, membrane damage, and accumulation of autofluorescent phenolics in lettuce cells challenged by Bremia lactucae. Plant J 9:851–865
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Buchter R, Stromberg A, Schmelzer E, Kombrink E (1997) Primary structure and expression of acidic (class II) chitinase in potato. Plant Mol Biol 35:749–761
Cabrefiga J, Montesinos E (2005) Analysis of aggressiveness of Erwinia amylovora using disease-dose and time relationships. Phytopathology 95:1430–1437
Caten CE, Jinks JL (1968) Spontaneous variability of single isolates of Phytophthora infestans. Can J Bot 46:329–348
Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 8:884–896
Cho M, Moinuddin SGA, Helms GL, Hishiyama S, Eichinger D, Davin LB, Lewis ND (2003) (+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata). Proc Natl Acad Sci USA 100:10641–10646
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3:371–390
Do PT, Prudent M, Sulpice R, Causse M, Fernie AR (2010) The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population. Plant Physiol 154:1128–1142
Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001) Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212:250–263
Ferrer JL, Austin MB, Stewart C Jr, Noel JP (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Phys Biochem 46:356–370
Fry W (2008) Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol 9:385–402
Gachomo EW, Seufferheld MJ, Kotchoni SO (2010) Melanization of appressoria is critical for the pathogenicity of Diplocarpon rosae. Mol Biol Rep 37:3583–3591
Haas BJ et al (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461:393–398
Hakimi SM, Krohn BM, Stark DM (2006) Monsanto Technology LLC. Method of imparting disease resistance to plants by reducing polyphenol oxidase activities. United States Patent US 7,122,719 B2
Halim VA, Eschen-Lippold L, Altmann S, Birschwilks M, Scheel D, Rosahl S (2007) Salicylic acid is important for basal defense of Solanum tuberosum against Phytophthora infestans. Mol Plant Microbe Interact 20:1346–1352
Hemaiswarya S, Soudaminikkutty R, Narasumani ML, Doble M (2011) Phenylpropanoids inhibit protofilament formation of Escherichia coli cell division protein FtsZ. J Med Microbiol 60:1317–1325
Hernandez-Romero D, Solano F, Sanchez-Amat A (2005) Polyphenol oxidase activity expression in Ralstonia solanacearum. Appl Environ Microbiol 71:6808–6815
Hoegen E, Stromberg A, Pihlgren U, Kombrink E (2002) Primary structure and tissue-specific expression of the pathogenesis-related protein PR-1b in potatodagger. Mol Plant Pathol 3:329–345
Huckelhoven R (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45:101–127
Jacobson ES (2000) Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717
Judelson HS, Tooley PW (2000) Enhanced polymerase chain reaction methods for detecting and quantifying Phytophthora infestans in plants. Phytopathology 90:1112–1119
Judelson HS, Tani S, Narayan RD (2009) Metabolic adaptation of Phytophthora infestans during growth on leaves, tubers and artificial media. Mol Plant Pathol 10:843–855
Kroner A, Hamelin G, Andrivon D, Val F (2011) Quantitative resistance of potato to Pectobacterium atrosepticum and Phytophthora infestans: integrating PAMP-triggered response and pathogen growth. PLOS ONE 6:e23331
La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heitz T (2004) Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev 198:267–284
Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PG (2009) Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol 150:1567–1575
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Liu Q, Paroo Z (2010) Biochemical principles of small RNA pathways. Annu Rev Biochem 79:295–319
Llorente B, Bravo-Almonacid F, Cvitanich C, Orlowska E, Torres HN, Flawia MM, Alonso GD (2010) A quantitative real-time PCR method for in planta monitoring of Phytophthora infestans growth. Lett Appl Microbiol 51:603–610
Llorente B et al (2011) Safety assessment of nonbrowning potatoes: opening the discussion about the relevance of substantial equivalence on next generation biotech crops. Plant Biotechnol J 9:136–150
Lou Z, Wang H, Zhu S, Ma C, Wang Z (2011) Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 76:M398–M403
Lyr H (1962) Detoxification of heartwood toxins and chlorophenols by higher fungi. Nature 195:289–290
Maher EA, Bate NJ, Ni W, Elkind Y, Dixon RA, Lamb CJ (1994) Increased disease susceptibility of transgenic tobacco plants with suppressed levels of preformed phenylpropanoid products. Proc Natl Acad Sci USA 91:7802–7806
Martinez E, Duvnjak Z (2006) Enzymatic degradation of chlorogenic acid using a polyphenol oxidase preparation from the white-rot fungus Trametes versicolor ATCC 42530. Process Biochem 41:1835–1841
Mayer AM (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67:2318–2331
McCurdy RD, McGrath JJ, Mackay-Sim A (2008) Validation of the comparative quantification method of real-time PCR analysis and a cautionary tale of housekeeping gene selection. Gene Ther Mol Biol 12:15–24
Montesano M, Hyytiainen H, Wettstein R, Palva ET (2003) A novel potato defence-related alcohol:NADP+ oxidoreductase induced in response to Erwinia carotovora. Plant Mol Biol 52:177–189
Montesano M, Brader G, Ponce de Leon I, Palva ET (2005) Multiple defence signals induced by Erwinia carotovora ssp. carotovora elicitors in potato. Mol Plant Pathol 6:541–549
Nakayama T et al (2000) Aureusidin synthase: a polyphenol oxidase homolog responsible for flower coloration. Science 290:1163–1166
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22:746–754
Nikitina VE, Vetchinkina EP, Ponomareva EG, Gogoleva YV (2010) Phenol oxidase activity in bacteria of the genus Azospirillum. Microbiology 79:327–333
Nowara D et al (2010) HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22:3130–3141
Orlowska E, Fiil A, Kirk HG, Llorente B, Cvitanich C (2012) Differential gene induction in resistant and susceptible potato cultivars at early stages of infection by Phytophthora infestans. Plant Cell Rep 31:187–203
Ozcelik B, Kartal M, Orhan I (2011) Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol 49:396–402
Payne G, Parks TD, Burkhart W, Dincher S, Ahl P, Metraux JP, Ryals J (1988) Isolation of the genomic clone for pathogenesis-related protein-1a from Nicotiana tabacum cv Xanthi-Nc. Plant Mol Biol 11:89–94
Payyavula RS, Navarre DA, Kuhl JC, Pantoja A, Pillai SS (2012) Differential effects of environment on potato phenylpropanoid and carotenoid expression. BMC Plant Biol 12:39
Payyavula RS, Navarre DA, Kuhl J, Pantoja A (2013) Developmental effects on phenolic, flavonol, anthocyanin, and carotenoid metabolites and gene expression in potatoes. J Agric Food Chem 61:7357–7365
Pinero S, Rivera J, Romero D, Cevallos MA, Martinez A, Bolivar F, Gosset G (2007) Tyrosinase from Rhizobium etli is involved in nodulation efficiency and symbiosis-associated stress resistance. J Mol Microbiol Biotechnol 13:35–44
Richter C, Dirks ME, Gronover CS, Prüfer D, Moerschbacher BM (2012) Silencing and heterologous expression of ppo-2 indicate a specific function of a single polyphenol oxidase isoform in resistance of dandelion (Taraxacum officinale) against Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 25:200–210
Rivero RM, Ruiz JM, Garcia PC, Lopez-Lefebre LR, Sanchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160:315–321
Rommens CM, Ye J, Richael C, Swords K (2006) Improving potato storage and processing characteristics through all-native DNA transformation. J Agric Food Chem 54:9882–9887
Shadle GL, Wesley SV, Korth KL, Chen F, Lamb C, Dixon RA (2003) Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of l-phenylalanine ammonia-lyase. Phytochemistry 64:153–161
Si-Ammour A, Mauch-Mani B, Mauch F (2003) Quantification of induced resistance against Phytophthora species expressing GFP as a vital marker: beta-aminobutyric acid but not BTH protects potato and Arabidopsis from infection. Mol Plant Pathol 4:237–248
Simon P (2003) Q-gene: processing quantitative real-time RT-PCR data. Bioinformatics 19:1439–1440
Steiner U, Oerke EC (2007) Localized melanization of appressoria is required for pathogenicity of Venturia inaequalis. Phytopathology 97:1222–1230
Steiner U, Schliemann W, Böhm H, Strack D (1999) Tyrosinase involved in betalain biosynthesis of higher plants. Planta 208:114–124
Sung WS, Lee DG (2010) Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl Chem 82:219–226
Thipyapong P, Hunt MD, Steffens JC (2004a) Antisense downregulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 220:105–117
Thipyapong P, Melkonian J, Wolfe DW, Steffens JC (2004b) Suppression of polyphenol oxidases increases stress tolerance in tomato. Plant Sci 167:693–703
Thygesen PW, Dry IB, Robinson SP (1995) Polyphenol oxidase in potato. A multigene family that exhibits differential expression patterns. Plant Physiol 109:525–531
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus “Phytoanticipins”. Plant Cell 6:1191–1192
Villarino M, Sandín-España P, Melgarejo P, De Cal A (2011) High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J Agric Food Chem 59:3205–3213
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20
Whisson SC, Avrova AO, Van West P, Jones JT (2005) A method for double-stranded RNA-mediated transient gene silencing in Phytophthora infestans. Mol Plant Pathol 6:153–163
Yang HY, Chen CW (2009) Extracellular and intracellular polyphenol oxidases cause opposite effects on sensitivity of Streptomyces to phenolics: a case of double-edged sword. PLOS ONE 4:e7462
Yao K, De Luca V, Brisson N (1995) Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 7:1787–1799
Yu D, Liu Y, Fan B, Klessig DF, Chen Z (1997) Is the high basal level of salicylic acid important for disease resistance in potato? Plant Physiol 115:343–349
Acknowledgments
We thank A. Andreu and F. Mauch for kindly providing the P. infestans and P. infestans-GFP strains, respectively. We thank C. Cvitanich and E. Orlowska for valuable experimental advice, and also M. A. Phillips, M. E. Segretin, and D. Caparrós-Ruiz for their critical analysis of the manuscript. This work was funded by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Facultad de Ciencias Exactas y Naturales (FCEyN), Universidad de Buenos Aires (UBA), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. Additional support was received from Coimbra Group and Wood-Whelan research fellowships from the International Union of Biochemistry and Molecular Biology (IUBMB).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Llorente, B., López, M.G., Carrari, F. et al. Downregulation of polyphenol oxidase in potato tubers redirects phenylpropanoid metabolism enhancing chlorogenate content and late blight resistance. Mol Breeding 34, 2049–2063 (2014). https://doi.org/10.1007/s11032-014-0162-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-014-0162-8