Abstract
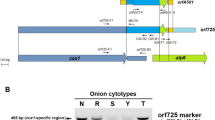
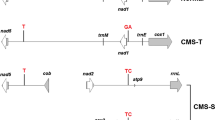
Molecular markers, coxII SCAR, atp6-2 SCAR and accD-U, have been used for marker-assisted selection of cytoplasmic male sterility (CMS) in pepper. However, the presence of these markers at the sub-stoichiometric level in maintainer lines affects the reliable selection of male sterile (S-) cytoplasm. This study aimed to develop a new CMS-specific molecular marker, SCAR130, for reliable identification of S-cytoplasm in pepper, while the new and three previous molecular markers were used to determine the cytoplasm types of pepper lines. Based on mitochondrial genome sequence related amplified polymorphism (SRAP) analysis of the CMS lines and the maintainer lines, SCAR130 was developed from a 10-bp deletion at the SRAP primer binding site in the CMS line (130 bp) compared with that in the maintainer line (140 bp). S-cytoplasm could be unambiguously selected from the pepper lines by the different length of the marker bands. Application of the four molecular markers to various pepper lines revealed that SCAR130 is more reliable than the other three previous markers, orf507, ψatp6-2 and accD-U. Homology alignment with BLAST showed that the marker was located between trnE and trnS in the Nicotiana tabacum mitochondrial genome. Furthermore, expression of the marker-linked gene was significantly higher at the pollen abortive stage in the CMS line (HW203A) than in the maintainer line, which indicated that the marker was closely related to male sterility. Hence, factors other than orf507 and ψatp6-2 may exist for the regulation of male sterility in pepper.




Similar content being viewed by others
References
Abad AR, Mehrtens BJ, Mackenzie SA (1995) Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated protein in cytoplasmic male sterile bean. Plant Cell 7:271–285
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G (1992) Sequence and transcript analysis of the Nco2.5 Ogura specific fragment correlated with cytoplasmic male-sterility in Brassica cybrids. Mol Gen Genet 235:340–348
Dieterich JH, Braun HP, Schmitz UK (2003) Alloplasmic male sterility in Brassica napus (CMS ‘Tournefortii-Stiewe’) is associated with a special gene arrangement around a novel atp9 gene. Theor Appl Genet 269:723–731
Eckardt NA (2007) Mitochondrial recombination surveillance. Plant Cell 19:1139
EU Arabidopsis Genome Sequencing Consortium and Cold Spring Harbor, Washington University in St. Louis, and the PE Biosystems Arabidopsis Sequencing Consortium (1999) Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391:485–488
Fang ZY, Sun PT, Liu YM et al (2001) Investigation of different types of male sterility and application of dominant male sterility in cabbage. China Veg 1:6–10
Gulyas G, Shin Y, Kim H, Lee JS, Hirata Y (2010) Altered transcript reveals an orf507 sterility-related gene in chili pepper (Capsicum annuum L.). Plant Mol Biol Rep 28:605–612
Hanson MR (1991) Plant mitochondrial mutation and male sterility. Annu Rev Genet 25:461–486
Hanson M, Bentolila S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16:154–169
Havey MJ (1993) A putative donor of S-cytoplasm and its distribution among open-pollinated populations of onion. Theor Appl Genet 86:128–134
Havey MJ (2000) Diversity among male-sterility-inducing and male-fertile cytoplasms of onion. Theor Appl Genet 101:778–782
Hernould M, Suharsono S (1993) Male-sterility induction in transgenic tobacco plants with an unedited atp9 mitochondrial gene from wheat. Proc Natl Acad Sci USA 90:2370–2374
Ji JJ, Huang W, Yin CC, Gong ZH (2013) Mitochondrial cytochrome c oxidase and F1F0-ATPase dysfunction in peppers (Capsicum annuum L.) with cytoplasmic male sterility and its association with orf507 and Ψatp6-2 genes. Int J Mol Sci 14:1050–1068
Jo YK, Jeong HJ, Kang BC (2009) Development of a CMS-specific marker based on chloroplast-derived mitochondrial sequence in pepper. Plant Biotechnol Rep 3:309–315
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin
Kim DH, Kim BD (2005) Development of SCAR markers for early identification of cytoplasmic male sterility genotype in chili pepper (Capsicum annuum L.). Mol Cells 20(3):416–422
Kim DH, Kim BD (2006) The organization of mitochondrial atp6 gene region in male fertile and CMS lines of pepper (Capsicum annuum L.). Curr Genet 49:59–67
Kim DH, Kang JG, Kim BD (2007a) Isolation and characterization of the cytoplasmic male sterility-associated orf456 gene of chili pepper (Capsicum annuum L.). Plant Mol Biol 63:519–532
Kim S, Lim H, Park S, Cho KH, Sung SK, Oh DG, Kim KT (2007b) Identification of a novel mitochondrial genome type and development of molecular markers for cytoplasm classification in radish (Raphanus sativus L.). Theor Appl Genet 115:1137–1145
Krishnasamy S, Makaroff CA (1993) Characterization of the radish mitochondrial orfB locus: possible relationship with male sterility in Ogura radish. Curr Genet 24:156–163
Krishnasamy S, Makaroff CA (1994) Organ-specific reduction in the abundance of a mitochondrial protein accompanies fertility restoration in cytoplasmic male-sterile radish. Plant Mol Biol 26:935–946
Kumar R, Kumar S, Dwivedi N, Kumar S, Rai A et al (2009) Validation of SCAR markers, diversity analysis of male sterile (S-) cytoplasms and isolation of an alloplasmic S-cytoplasm in Capsicum. Sci Hort 120:167–172
Li GQ (2011) Investigation of the characteristics of floral organ in male sterile pepper and cloning of CaCP26 in pepper and its analysis. Dissertation, Northwest A&F University
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103:455–461
Li Z, Pan JS, Guan Y, Tao QY, He HL et al (2008) Development and fine mapping of three co-dominant SCAR markers linked to the M/m gene in the cucumber plant (Cucumis sativus L.). Theor Appl Genet 117:1253–1260
Lin X, Kaul S, Rounsley S, Shea T, Benito MI et al (1999) Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402:761–767
Liu YG, Robert FW (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragment from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Liu ZY, Peter SO, Long M, Weingartner U, Stamp P, Kaeser O (2002) A PCR assay for rapid discrimination of sterile cytoplasm types in maize. Crop Sci 42:566–569
Ma Y, Huang W, Ji JJ, Gong ZH, Yin CC, Ahmed SS, Zhao ZL (2013) Maintaining and restoration cytoplasmic male sterility systems in pepper (Capsicum annuum L.). Genet Mol Res 12(3):2320–2331. doi:10.4238/2013.January.4.8
Makaroff CA, Palmer JD (1988) Mitochondrial DNA rearrangements and transcriptional alterations in the male-sterile cytoplasm of Ogura radish. Mol Cell Biol 8:1474–1490
Makaroff CA, Apel IJ, Palmer JD (1991) The role of cox2-associated repeated sequences in plant mitochondrial DNA rearrangements and radish cytoplasmic sterility. Curr Genet 19(3):183–190
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease resistance genes by bulkes segregant analysis: a rapid method to detect markers in specific genomic regions using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Millar AH, Liddell A, Leaver CJ (2007) Isolation and subfractionation of mitochondria from plants. Method Cell Biol 80:65–90
Min W (2009) Molecular genetic analysis and allelic discrimination of the restorer-of-fertility (Rf) gene in peppers (Capsicum annuum L.). Dissertation, Seoul National University
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Negi MS, Devic M, Delsny M, Lakshmikumaram M (2000) Identification of AFLP fragments linked to seed color coat in Brassica juncea and conversion to a SCAR marker for rapid selection. Theor Appl Genet 101:146–152
Panaud O, Chen XL, McCouch SR (1996) Development microsatellite markers characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Peterson PA (1958) Cytoplasmically inherited male sterility in Capsicum. Am Nat 92:111–119
Quigley F, Weil JH (1985) Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet 9:495–503
San Mauro D, Gower DJ, Oommen OV, Wilkinson M, Zardoya R (2004) Phylogeny of caecilian amphibians (gymnophiona) based on complete mitochondrial genomes and nuclear RAG1. Mol Phylogenet Evol 33(2):413–427
Sane AP, Seth P, Ranade SA, Nath P, Sane PV (1997) RAPD analysis of isolated mitochondrial DNA reveals heterogeneity in elite wild abortive (WA) cytoplasm in rice. Theor Appl Genet 95:1098–1103
Sato Y (1998) PCR amplification of CMS-specific mitochondrial nucleotide sequences to identify cytoplasmic genotypes of onion (Allium cepa L.). Theor Appl Genet 96:367–370
Satoh M, Kubo T, Nishizawa S, Estiati A, Itchoda N et al (2004) The cytoplasmic male-sterile type and normal type mitochondrial genomes of sugar beet share the same complement of genes of known function but differ in the content of expressed ORFs. Mol Gen Genomics 272:247–256
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Siculella L, Palmer JD (1988) Physical and gene organization of mitochondrial DNA in fertile and male-sterile sunflower. CMS-associated alterations in structure and transcription of the atpA gene. Nucleic Acids Res 16:3787–3799
Ullstrup AJ (1972) The impact of the southern corn leaf blight of epidemics 1970–1971. Annu Rev Phytopathol 10:37–50
Virmani SS, Aquino RC, Khush GS (1982) Heterosis breeding in rice (Oryza sativa L.). Theor Appl Genet 63:373–380
Wang QB, Zhang YY (2012) Chloroplast and mitochondrial SSR help to distinguish allo-cytoplasmic male sterile types in cabbage (Brassica oleracea L. var. capitata). Mol Breed 30:709–716
Wintz H, Chen HC, Sutton CA, Conley CA, Cobb A, Ruth D, Hanson MR (1995) Expression of the CMS-associated urfS sequence in transgenic petunia and tobacco. Plant Mol Biol 28:83–92
Wu ZM, Hu KL, Wang DY (2007) AFLP analysis of mitochondrial DNA from a cytoplasmic male sterility line and its maintainer in pepper (Capsicum annuum L.). Mol Plant Breed 5(2):261–262
Xia XH (2005) Mutation and selection on the anticodon of tRNA genes in vertebrate mitochondrial genomes. Gene 345(1):13–20
Yi B, Chen Y, Lei S, Tu J, Fu T (2006) Fine mapping of the recessive genic male-sterile gene (Bnms1) in Brassica napus L. Theor Appl Genet 113:643–650
Yoo IW (1990) The inheritance of male sterility and its utilization for breeding in pepper (Capsicum spp.). Kyung Hee University, South Korea, Ph. D. Dissertation
Zhang YL, Jia QL, Li DW, Wang JE, Yin YX, Gong ZH (2013) Characteristic of the pepper CaRGA2 gene in defense responses against Phytophthora capsici Leonian. Int J Mol Sci 14(5):8985–9004
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31272163), “The Twelfth Five-Year” Plan of National Science and Technology in Rural Areas (No. 2011BAD12B03), the Shaanxi Provincial Science and Technology Coordinating Innovative Engineering Project (2012KTCL02-09) and the Northwest A&F University Cyrus Tang Seed Development Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiao-Jiao Ji and Wei Huang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ji, JJ., Huang, W., Yin, YX. et al. Development of a SCAR marker for early identification of S-cytoplasm based on mitochondrial SRAP analysis in pepper (Capsicum annuum L.). Mol Breeding 33, 679–690 (2014). https://doi.org/10.1007/s11032-013-9984-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-9984-z