Abstract
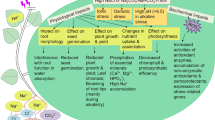
Abiotic stress, such as salt, high light intensity and extreme temperature, can result in enhanced production of reactive oxygen species (ROS). One of the major ROS-scavenging enzymes of plants is peroxiredoxin (Prx). Peroxiredoxin Q (PrxQ), a member of the Prx gene family, was recently cloned from plants. To investigate the protective role of PrxQ during abiotic stress, we increased the capacity for its biosynthesis in Eustoma grandiflorum Shinn by overexpression of the PrxQ gene (SsPrxQ) from Suaeda salsa. The SsPrxQ gene driven by CaMV 35S promoter was expressed via E. grandiflorum. The rPrxQ protein shows antioxidant activity and thioredoxin-dependent peroxidase activity in vitro. Additionally, overexpression of SsPrxQ in E. grandiflorum leads to an increase in salt and high light intensity tolerance. These results indicate that SsPrxQ might act as an oxidative stress defensive gene in plants and could be useful for engineering stress-resistant plants.





Similar content being viewed by others
References
Broin M, Cuiné S, Eymery F, Rey P (2002) The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell 14(6):1417–1432. doi:10.1105/tpc.001644
Chen Y-T, Fang Q-S, Chiang C-H, Yeh S-D, Wu H-W, Yu T-A (2010) Transgenic Eustoma grandiflorum expressing the bar gene are resistant to the herbicide Basta. Plant Cell Tiss Org 102(3):347–356. doi:10.1007/s11240-010-9739-z
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57(5):779–795. doi:10.1007/s000180050041
Dietz K-J (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54(1):93
Dietz KJ, Horling F, König J, Baier M (2002) The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot 53(372):1321–1329. doi:10.1093/jexbot/53.372.1321
Eltayeb A, Kawano N, Badawi G, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225(5):1255–1264. doi:10.1007/s00425-006-0417-7
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17(7):1866–1875. doi:10.1105/tpc.105.033589
Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G (1997) Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta 203(4):460–469. doi:10.1007/s004250050215
Hall A, Karplus PA, Poole LB (2009) Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J 276(9):2469–2477. doi:10.1111/j.1742-4658.2009.06985.x
Hosoya-Matsuda N, Motohashi K, Yoshimura H, Nozaki A, Inoue K, Ohmori M, Hisabori T (2005) Anti-oxidative stress system in cyanobacteria. J Biol Chem 280(1):840–846. doi:10.1074/jbc.M411493200
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. BBA-Bioenergetics 1787(1):3–14. doi:10.1016/j.bbabio.2008.09.013
Jing L-W, Chen S-H, Guo X-L, Zhang H, Zhao Y-X (2006) Overexpression of a chloroplast-located peroxiredoxin Q Gene, SsPrxQ, increases the salt and low-temperature tolerance of Arabidopsis. J Integ Plant Biol 48(10):1244–1249. doi:10.1111/j.1744-7909.2006.00357.x
Kiba A, Nishihara M, Tsukatani N, Nakatsuka T, Kato Y, Yamamura S (2005) A peroxiredoxin Q homolog from gentians is involved in both resistance against fungal disease and oxidative stress. Plant Cell Physiol 46(6):1007–1015. doi:10.1093/pcp/pci109
Lamkemeyer P, Laxa M, Collin V, Li W, Finkemeier I, Schöttler MA, Holtkamp V, Tognetti VB, Issakidis-Bourguet E, Kandlbinder A, Weis E, Miginiac-Maslow M, Dietz K-J (2006) Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J 45(6):968–981. doi:10.1111/j.1365-313X.2006.02665.x
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Lester Packer RD (ed) Methods in enzymology, vol 148. Academic Press, New York, pp 350–382
Meneguzzo S, Navam-Izzo F, Izzo R (1999) Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. J Plant Physiol 155(2):274–280. doi:10.1016/s0176-1617(99)80019-4
Pan H, Yang C-P, Wei Z-G, Jiang J (2006) DNA extraction of birch leaves by improved CTAB method and optimization of its ISSR system. J For Res 17(4):298–300. doi:10.1007/s11676-006-0068-3
Prashanth S, Sadhasivam V, Parida A (2008) Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res 17(2):281–291. doi:10.1007/s11248-007-9099-6
Rouhier N, Jacquot J-P (2005) The plant multigenic family of thiol peroxidases. Free Radical Bio Med 38(11):1413–1421. doi:10.1016/j.freeradbiomed.2004.07.037
Rouhier N, Gelhaye E, Jacquot JP (2002) Glutaredoxin-dependent peroxiredoxin from poplar. J Biol Chem 277(16):13609–13614. doi:10.1074/jbc.M111489200
Rouhier N, Gelhaye E, Gualberto JM, Jordy M-N, De Fay E, Hirasawa M, Duplessis S, Lemaire SD, Frey P, Martin F, Manieri W, Knaff DB, Jacquot J-P (2004) Poplar peroxiredoxin Q, a thioredoxin-linked chloroplast antioxidant functional in pathogen defense. Plant Physiol 134(3):1027–1038. doi:10.1104/pp.103.035865
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41(11):1229–1234. doi:10.1093/pcp/pcd051
Sekmen AH, Türkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Planta 131(3):399–411
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161(3):613–619. doi:10.1016/s0168-9452(01)00450-2
Sumithra K, Jutur P, Carmel B, Reddy A (2006) Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul 50(1):11–22. doi:10.1007/s10725-006-9121-7
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11(7):1195–1206. doi:10.1105/tpc.11.7.1195
Wu W, Ji J, Wang G, Zhao Q, Jin C, Guan C, Josine T (2012) Overexpression of AtchyB in Eustoma grandiflorum Shinn enhances its tolerance to high-light via zeaxanthin accumulation. Plant Mol Bio Rep 30:1–11. doi:10.1007/s11105-012-0460-4
Acknowledgments
This work was supported by the National Genetically Modified Organism Major Projects of China: Breeding of Transformed Maize with Higher Nutrient Absorption Efficiency (2011ZX08003-005), National Natural Science Foundation of China (31271793) and 2010 PhD supervisor Doctoral Program of Higher Specialized Research Fund (20100032110060).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, C., Liu, X., Song, X. et al. Overexpression of a peroxiredoxin Q gene, SsPrxQ, in Eustoma grandiflorum Shinn enhances its tolerance to salt and high light intensity. Mol Breeding 33, 657–667 (2014). https://doi.org/10.1007/s11032-013-9982-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-9982-1