Abstract
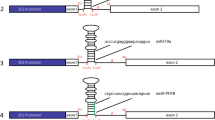
The effect of introns on silencing efficiency was tested in inverted repeat constructs of Granule-Bound Starch Synthase (GBSSI) cDNA by comparing the silencing efficiencies induced by inverted repeat constructs with and without introns. No effect could be attributed to the presence of introns indicating that the introns neither enhance nor inhibit post-transcriptional gene silencing. The effect of a spliceable intron in the spacer was studied by comparing constructs harbouring a spliceable or a non-spliceable intron in the spacer. As opposed to the general belief that splicing of an intron increases silencing efficiency, the use of a spliceable intron in the spacer did not result in enhancement of silencing in our experimental system.
Similar content being viewed by others
Abbreviations
- dsRNA:
-
double-stranded RNA
- GBSSI:
-
Granule-Bound Starch Synthase I
- IME:
-
intron-mediated enhancement
- PTGS:
-
Post-Transcriptional Gene Silencing
- siRNA:
-
small interfering RNA
References
Bonifacio G.F., Brown T., Conn G.L., Lane A.N. (1997). Comparison of the electrophoretic and hydrodynamic properties of DNA and RNA oligonucleotide duplexes. Biophys. J. 73:1532–1538
Callis J., Fromm M., Walbot V. (1987). Introns increase gene expression in cultured maize cells. Genes Dev. 1:1183–1200
Cerutti H. (2003). RNA interference: traveling in the cell and gaining functions? Trends Genet. 19:39–46
Chambers S.P., Prior S.E., Barstow D.A., Minton N.P. (1988). The pMTL nic- cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68:139–149
Dean C., Favreau M., Bond-Nutter D., Bedbrook J., Dunsmuir P. (1989). Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes. Plant Cell 1:201–208
Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Goldoni M., Azzalin G., Macino G., Cogoni C. (2004). Efficient gene silencing by expression of double stranded RNA in Neurospora crassa. Fungal Genet. Biol. 41:1016–1024
Hamilton A.J., Baulcombe D.C. (1999). A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286:950–952
Hamilton A., Voinnet O., Chappell L., Baulcombe D. (2002). Two classes of short interfering RNA in RNA silencing. EMBO J. 21:4671–4679
Heeres P., Schippers-Rozenboom M., Jacobsen E., Visser R.G.F. (2002). Transformation of a large number of potato varieties: genotype-dependent variation in efficiency and somaclonal variability. Euphytica 124:13–22
Heilersig H.J.B., Loonen A., Bergervoet M., Wolters A.M.A. and Visser R.G.F. 2006. Post-transcriptional gene silencing in potato: effects of size and sequence of the inverted repeats. Plant Mol. Biol. (in press).
Hendriks T., Vreugdenhil D., Stiekema W.J. (1991). Patatin and four serine proteinase inhibitor genes are differentially expressed during potato tuber development. Plant Mol. Biol. 17:385–394
Hofacker I.L. (2003). Vienna RNA secondary structure server. Nucleic Acids Res. 31:3429–3431
Hofvander P., Persson P.T., Tallberg P.T. and Wikstroem O. 1992. Genetically engineered modification of potato from amylopectin-type starch. International Patent Application WO 92/11376
Kuipers A.G.J., Jacobsen E., Visser R.G.F. (1994). Formation and deposition of amylose in the potato tuber starch granule are affected by the reduction of granule-bound starch synthase gene expression. Plant Cell 6:43–52
Kuipers A.G.J., Soppe W.J.J., Jacobsen E., Visser R.G.F. (1995). Factors affecting the inhibition by antisense RNA of granule-bound starch synthase gene expression in potato. Mol. Gen. Genet. 246:745–755
Lazo G.R., Stein P.A., Ludwig R.A. (1991). A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/Technology 9:963–967
Luehrsen K.R., Walbot V. (1991). Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol. Gen. Genet. 225:81–93
Maas C., Laufs J., Grant S., Korfhage C., Werr W. (1991). The combination of a novel stimulatory element in the first exon of the maize Shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold. Plant Mol. Biol. 16:199–207
Mascarenhas D., Mettler I.J., Pierce D.A., Lowe H.W. (1990). Intron-mediated enhancement of heterologous gene expression in maize. Plant Mol. Biol. 15:913–920
McGinnis K., Chandler V., Cone K., Kaeppler H., Kaeppler S., Kerschen A., Pikaard C., Richards E., Sidorenko L., Smith T., Springer N., Wulan T. (2005). Transgene-induced RNA interference as a tool for plant functional genomics. Methods Enzymol. 392:1–24
Miki D., Itoh R., Shimamoto K. (2005). RNA silencing of single and multiple members in a gene family of rice. Plant Physiol. 138:1903–1913
Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497
Nakayashiki H., Hanada S., Nguyen B.Q., Kadotani N., Tosa Y., Mayama S. (2005). RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 42:275–283
Rethmeier N., Seurinck J., Van Montagu M., Cornelissen M. (1997). Intron-mediated enhancement of transgene expression in maize is a nuclear, gene-dependent process. Plant J. 12:895–899
Rose A.B. (2002). Requirements for intron-mediated enhancement of gene expression in Arabidopsis. RNA 8:1444–1453
Sambrook J., Fritsch E.F., Maniatis T. (1989). Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, Cold Spring Harbor Laboratory Press
Smith N.A., Singh S.P., Wang M.B., Stoutjesdijk P.A., Green A.G., Waterhouse P.M. (2000). Total silencing by intron-spliced hairpin RNAs. Nature 407:319–320
Takken F.L.W., Luderer R., Gabriëls S.H.E.J., Westerink N., Lu R., de Wit P.J.G.M., Joosten M.H.A.J. (2000). A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J. 24:275–283
van der Leij F.R., Visser R.G.F., Ponstein A.S., Jacobsen E., Feenstra W.J. (1991). Sequence of the structural gene for granule-bound starch synthase of potato (Solanum tuberosum L.) and evidence for a single point deletion in the amf allele. Mol. Gen. Genet. 228:240–248
Visser R.G.F., Hergersberg M., van der Leij F.R., Jacobsen E., Witholt B., Feenstra W.J. (1989). Molecular cloning and partial characterization of the gene for granule-bound starch synthase from a wild type and an amylose-free potato (Solanum tuberosum L.). Plant Sci. 64:185–192
Visser R.G.F., Somhorst I., Kuipers G.J., Ruys N.J., Feenstra W.J., Jacobsen E. (1991). Inhibition of the expression of the gene for granule-bound starch synthase in potato by antisense constructs. Mol. Gen. Genet. 225:289–296
Wang H., Lee M.M., Schiefelbein J.W. (2002). Regulation of the cell expansion gene RHD3 during Arabidopsis development. Plant Physiol. 129:638–649
Wesley S.V., Helliwell C.A., Smith N.A., Wang M.B., Rouse D.T., Liu Q., Gooding P.S., Singh S.P., Abbott D., Stoutjesdijk P.A., Robinson S.P., Gleave A.P., Green A.G., Waterhouse P.M. (2001). Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 27:581–590
Zhang H., Kolb F.A., Brondani V., Billy E., Filipowicz W. (2002) Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21:5875–5885
Acknowledgements
This work was supported by the European Union (project QLK3-2000-00078). We would like to thank Mark Fiers for his help with the in silico predictons of the dsRNA structures, and Marcos Malosetti for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heilersig, B., Loonen, A., Wolters, AM. et al. Presence of an intron in inverted repeat constructs does not necessarily have an effect on efficiency of post-transcriptional gene silencing. Mol Breeding 17, 307–316 (2006). https://doi.org/10.1007/s11032-006-9001-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-006-9001-x