Abstract
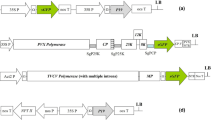
An approach that enables the increase of the quantity of a specific amino acid in crop plants is reported. Oleosin gene from Arabidopsis thaliana or 30K movement protein gene of Tobacco mosaic virus (TMV; genus Tobamovirus) were cloned under the control of napin or hybrid promoters, and in fusion to synthetic poly-histidine (poly-His) sequences for transformation into spring turnip rape (Brassica rapa subsp. oleifera; synonym to B. campestris). The most stable expression cassettes for the poly-His production prior to the plant transformation were selected by analyzing the protein expression in in vitro translation and in transient plant expression systems using GFP as marker. Expression of the poly-His-constructs in transgenic Brassica rapa plants was analyzed using dot and western blotting and PCR. The constructs were stably expressed in the third generation of the transgenic plant lines. Histidine content was measured from the seeds of the transgenic plants, and some plant lines had more than 20% increase in histidine content compared to wild type. The methodology may be widely applicable to increase the content of any amino acid in crop plants including those encoded by rare codons.
Similar content being viewed by others
References
Altenbach S.B., Kuo C., Staraci L.C., Pearson K.W., Wainwright C., Georgescu A. and Townsend J. 1992. Accumulation of a Brazil nut albumin in seeds of transgenic canola results in enhanced levels of seed protein methionine. Plant Mol Biol. 18: 235–45.
Azevedo R.A. 2001. Analysis of the aspartic acid metabolic pathway using mutant genes. Amino Acids 22: 217–230.
BrinchPedersen H., Galili G., Knudsen H. and Holm P.B. 1996. Engineering of the aspartate family biosynthetic pathway in barley (Hordeum vulgare L.) by transformation with heterologous genes encoding feed-back - insensitive aspartate kinase and dihydrodipicolinate synthase. Plant Mol. Biol. 32: 611–620.
Carnici P. and Hayashizaki Y. 1999. High-efficiency full-length cDNA cloning. Method Enzymol. 303: 19–44.
De Clercq A., Vandewiele M., Van Damme J., Guerche P., Van Montagu M., Vandekerckhove J. and Krebbers E. 1990. Stable accumulation of modified 2S albumin seed storage proteins with higher methionine contents in transgenic plants. Plant Physiol. 94: 970–979.
European Comission Directive 98/64/ EC. 1998. Community methods of analysis for the determination of amino acids, crude oils and fats, and olaquindox in feedstuffs and amending directive 71/393/EEC. Off. J. Eur. Communities 257: 4–23.
Heim R., Cubitt A.B. and Tsien R.Y. 1995. Improved green fluorescence. Nature 373: 663–664.
Keeler S.J., Maloney C.L., Webber P.Y., Patterson C., Hirata L.T., Falco S.C. and Rice J.A. 1997. Expression of de novo high-lysine alpha-helical coiled-coil proteins may significantly increase the accumulated levels of lysine in mature seeds of transgenic tobacco plants. Plant Mol. Biol. 34: 15–29.
Kim C.H., Choung J.J. and Chamberlain D.G. 2000. Variability in the ranking of the three most-limiting amino acids for milk protein production in dairy cows consuming grass silage and a cereal-based supplement containing feather meal. J. Sci. Food. Agric. 80: 1386–1392.
Korhonen M., Vanhatalo A. and Huhtanen P. 2000. Responses to graded postruminal doses of histidine in dairy cows fed grass silage diets. J. Dairy Sci. 83: 1533–1545.
Korhonen M., Vanhatalo A. and Huhtanen P. 2002. Evaluation of isoleucine, leucine and valine as a second limiting amino acid for milk production in dairy cows fed grass silage diet. J. Dairy Sci. 85: 1533–1545.
Lee G.H., Kang H., Kim D.H. and Park W.J. 2001. Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J. Biol. Chem. 276: 39533–39538.
Mertz E.T., Bates L.S and Nelson O.E. 1964. Mutant gene that changes protein composition and increase lysine content of maize endosperm. Science 145: 279–280.
Molvig L., Tabe L.M., Eggum B.O., Moore A.E., Stuart C., Spencer D. and Higgins T.J.V. 1997. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc. Natl. Acad. Sci. USA 94: 8393–8398.
Murphy D.J. 1993. Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Progr. Lipid Res. 32: 247–280.
National Research Council. 2001. Nutrient requirements of dairy cattle, Seventh revised edition. National Academy Press, Washington, DC, USA, pp. 1–230.
Padgett H.S., Epel B.L., Kahn Th.W., Heinlein M., Watanabe Y. and Beachy R.N. 1996. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J. 10: 1079–1088.
Parmenter D.L., Boothe J.G., van Rooijen G.J.H., Yeung E.C. and Moloney M.M. 1995. Production of biologically active hirudin in plant seeds using oleosin partitioning. Plant Mol. Biol. 29: 1167–1180.
Rulguin H. and Verite R. 1993. Amino acid nutrition of dairy cows: productive effects and animal requirements.. In: Garnsworthy P.C. and Cole D.J.A. (eds), recent advances in animal nutrition. Nottingham university press, Nottingham, UK, pp. 55–77.
Tomb J- F., White O., Kerlavage A.R., Clayton R.A., Sutton G.G., Fleischmann R.D., Ketchum K.A., Klenk H.P., Gill S., Dougherty B.A., Nelson K., Quackenbush J., Zhou L., Kirkness E.F., Peterson S., Loftus B., Richardson D., Dodson R., Khalak H.G., Glodek A., McKenney K., Fitzegerald L.M., Lee N., Adams M.D. and Venter J.C. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388: 593–547.
Töpfer R., Matzeit V., Gronenborn B., Schell J. and Steinbiss H.H. 1987. A set of plant expression vectors for transcriptional and translational fusions. Nucl. Acid Res. 15: 5890.
Varvikko T., Vanhatalo A., Jalava T. and Huhtanen P. 1999. Lactation and metabolic responses to graded abomasal doses of methione and lysine in dairy cows fed grass silage diets. J. Dairy Sci. 82: 2659–2673.
Wahlroos T., Soukka J., Denesyuk A., Wahlroos R., Korpela T. and Kilby N.J. 2003a. Oleosin expression and trafficking during oil body biogenesis in tobacco leaf cells. Genesis. 35: 125–32.
Wahlroos T., Susi P., Tylkina L., Malyshenko S., Zvereva S. and Korpela T. 2003b. Agrobacterium-mediated transformation and stable expression of the green fluorescent protein in Brassica rapa. Plant Physiol. Biochem. 42: 773–778.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahlroos, T., Susi, P., Solovyev, A. et al. Increase of histidine content in Brassica rapa subsp. oleifera by over-expression of histidine-rich fusion proteins. Mol Breeding 14, 455–462 (2005). https://doi.org/10.1007/s11032-005-0902-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11032-005-0902-x