Abstract
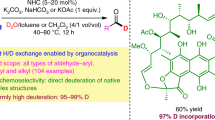
The concept of deuterium enrichment has gained more attention due to its advantages in the studies of clinical pharmacokinetics and metabolic profiles. In addition, it is cost and time efficient to develop deuterium-enriched drugs. Herein we built a combinatorial library of deuterated (S)-oxybutynins which all 8 D-compounds were characterized by MS, \(^{1}\hbox {H}\) NMR and \(^{ 13}\)C NMR.



Similar content being viewed by others
References
Elsig JSJ, Leuenberger D, Schneider REM, Leuenberger MJF, Fischer H, Stocker TF (2009) Stable isotope constraints on holocene carbon cycle changes from an antarctic ice core. Nature 461:507–510. doi:10.1038/nature08393
Radajewski S, Ineson P, Parekh NR, Murrell JC (2000) Stable-isotope probing as a tool in microbial ecology. Nature 403:646–649. doi:10.1038/35001054
Thomas GG (2014) Using deuterium in drug discovery: leaving the label in the drug. J Med Chem 57:3595–3611. doi:10.1021/jm4007998
Di CL, Moulin M, Haertlein M, Meilleur F, Christianson DW (2007) Expression, purification, assay and crystal structure of perdeuterated human arginase I. Arch Biochem Biophys 465:82–89. doi:10.1016/j.abb.2007.04.036
Nelson SD, Trager WF (2003) The use of deuterium isotope effects to probe the active site properties, mechanism of cytochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab Dispos 31:1481–1498. doi:10.1124/dmd.31.12.1481
Bell RP (1974) Liversidge lecture. Recent advances in the study of kinetic hydrogen isotope effects. Chem Soc Rev 3:513–544. doi:10.1039/cs9740300513
Samis HV, Baird MB, Massie HR (1974) Deuterium oxide effect on temperature-dependent survival in populations of Drosophila melanogaster. Science 183:427–427. doi:10.1126/science.183.4123.427
Foster AB (1985) Deuterium isotope effects in the metabolism of drugs and xenobiotics: implications for drug design. Adv Drug Res 14:1–40
Tung R (2010) The development of deuterium-containing drugs. Innovat Pharmaceut Tech 32:24–26, 28. Doi can’t be found from SciFinder and the website of Innovations in Pharmaceutical Technology
Uttamsingh V, Wells D, Soergel D (2009) CTP-347, a deuterated paroxetine analog exhibits reduced mechanism-based inactivation of CYP2D6 in healthy women. In: Presented at the 38th American College of Clinical Pharmacology
Sabounjian L, Shipley J, Braman V, Harnett M, Wu L, Turnquist D, Graham P (2012) Design and rationale for randomized, double-blind, placebo-controlled phase 2 study to evaluate the safety and efficacy of CTP-499 in patients with diabetic nephropathy (DN). Adv Chronic Kidney Dis 19:123. doi:10.1053/j.ackd.2012.02.007
Reitz AB, Gupta SK, Y Huang, Parker MH, Ryan RR (2007) The preparation and human muscarinic receptor profiling of oxybutynin and N-desethyloxybutynin enantiomers. Med Chem 3:543–545. doi:10.2174/157340607782360353
Anthony WC (2008) Deuterium-enriched oxybutynin. US 2008299219
Thomas GG, Sepehr S (2009) Substituted phenylcyclohexylglycolates. US 2009247628
Aberg G, McCullough JR (1996) Methods and compositions for treating urinary incontinence using optically pure (S)-oxybutynin. US 5532278
Aberg G, McCullough JR (1998) Methods and compositions for treating urinary incontinence using optically pure (S)-oxybutynin. US 5736577
Vandenbossche CP, de Croos P, Singh SP, Bakale RP, Wagler TR (2010) Formation of (S)-5-cyclohexyl-5-phenyl-1,3-dioxolane-2,4-dione: a key intermediate in the synthesis of (S)-oxybutynin hydrochloride. Org Process Res Dev 14:921–925. doi:10.1021/op100021w
Jerussi TP (2001) Methods for treatment of asthma using S-oxybutynin. US 6294582
Bakale RP, Lopez JL, Mcconville FX, Vandenbossche CP, Senanayake CH (2000) Synthesis of optically active cyclohexylphenylglycolate esters. US 6140529
Bakale RP, Lopez JL, Mcconville FX, Vandenbossche CP, Senanayake CH (1999) Carbonate Intermediates useful in the preparation of optically active cyclohexylphenylglycolate esters. US 5973182
Bakale RP, Lopez JL, Mcconville FX, Vandenbossche CP, Senanayake CH (2000) Synthesis of optically active cyclohexylphenylglycolic acid and its esters. WO 0023414
Campbell KN, Majewski RF (1965) Substituted aminobutynyl acetates. US 3176019
Majewski RF, Campbell KN, Dykstra S, Covington R, Simms JC (1965) Anticholinergic agents-esters of 4-dialkyl(or 4-polymethylene)amino-2-butynols. J Med Chem 8:719–720. doi:10.1021/jm00329a044
Adams TC, Dupont AC, Carter JP, Kachur JF, Guzewska ME, Rzeszotarski WJ, Farmer SG, Noronha-Blob L, Kaiser C (1991) Aminoalkynyldithianes. A new class of calcium channel blockers. J Med Chem 34:1585–1593. doi:10.1021/jm00109a010
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, F., Jiang, W., Czarnik, A.W. et al. Combinatorial synthesis of deuterium-enriched (S)-oxybutynin. Mol Divers 20, 605–610 (2016). https://doi.org/10.1007/s11030-016-9660-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9660-4