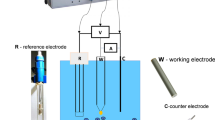
The operating principle and composition of the VNIIFTRI coulometric device is based on a three-chamber electrochemical cell of vertical construction that is part of the set of primary state standards for the pH scale and indices of pX ion indices. Metrological characteristics of the device ensure reproducibility of pH in the standard with an error of not more than 0.003 in the range of pH = 1.0–12, and this corresponds to the best world analogs.
Similar content being viewed by others
References
I. L. Knunyants (ed.), Large Encyclopaedic Dictionary. Chemistry [in Russian], BSE, Moscow (1998), p. 292.
M. Mariassy, K. W. Pratt, and P. Spitzer, “Major application of electrochemical techniques at national metrology institutes,” Metrologia, 46, 199213 (2009).
CODATA 2006, http://physics.nist.gov/cgi-bin/cuu/Value?f.
B. Taylor,V. Parker, and D. Landenberg, Fundamental Constants and Quantum Electrodynamics [Russian translation], Atomizdat, Moscow (1972).
K. W. Pratt, “Automated, high-precision coulometric titrimetry,” Analytica Chimica Acta, 289, 125 (1964).
M. Breitenbach, Precision Constant Current Coulometry System, http://www.bam.de/en/kompetenzen/pruefeinrichtungen_geraete/index.htm.
M. Máriássy, L. Vyskocil, and A. Mathiasová, “Link to the SI via primary direct methods,” Accred. Qual. Assur., No. 5, 437 (2000).
“Measurement of pH. Definition, standards and procedures (UPAC Recommendations 2002),” Pure Appl. Chem., 74, 2169 (2002).
A. S. Doinikov et al., “Commentary on the introduction of interstate standard GOST 8.134-98 Aqueous Solution pH scale,” Elektrokhimiya, 36, No. 3, 374 (2000).
O. V. Karpov, “VNIIFTRI standard base in the field of physicochemical measurements,” Proc. 3rd Int. Sci.-Pract. Conf. Khimmet-3–2008, Kiev (2008).
O. V. Karpov, V. D. Kutovoi, and V. A. Zvezdina, “State primary standard of pX ion activity indices in aqueous solutions,” ibid.
V. E. Bower and R. S. Davis, “The electrochemical equivalent of pure silver: a value of the Faraday constant,” J. Res. NBS, 85, No. 3, 175 (1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 4, pp. 13–15, April, 2010.
Rights and permissions
About this article
Cite this article
Artemenkov, M.A., Karpov, O.V., Kutovoi, V.D. et al. Coulometric device within a unit for state primary standards. Meas Tech 53, 368–372 (2010). https://doi.org/10.1007/s11018-010-9512-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-010-9512-z