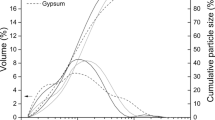
Studies are made of the use of hydrochloric acid for the autoclave decomposition of boehmite-kaolinite bauxites from the North Onezhky Deposit. The spatial location and structure of the bauxites are investigated, and the solid residue left after autoclave decomposition is examined by x-ray phase analysis and electron microscopy to study the mechanism of the bauxites’ dissolution. The data that is obtained confirms the presence of hard films of amorphous silica. These films prevent complete dissolution of the aluminum-bearing minerals: boehmite and kaolinite.









Similar content being viewed by others
References
A. S. Senyuta, A. V. Panov, A. G. Suss, and Yu. A. Layner, “Innovative technology for alumina production from low-grade raw materials,” Light Metals’2013, pp. 201–208, DOI: 101002/9781118663189.ch36.
A. G. Suss, A. A. Damaskin, A. S. Senyuta, et al., “The influence of the mineral composition of low-grade aluminum ores on aluminum extraction by acid leaching,” Light Metals’2014, pp. 105–109, DOI: 10.1002/9781118888438.ch18.
C. Knudsen, J. Wanvik, and H. Svahnberg, “Anorthosites in Greenland: a possible raw material for aluminum?” Geolog. Survey of Denmark and Greenland Bull., No. 26, 53–56 (2012).
R. Budro, S. Aleks, and F. Bazotto, Patent No. 2471010 RF, IPC C22BC21/00, “Method of extracting aluminum and iron from aluminous rocks,” subm. 05.07.2008, publ. 12.27.2012, Byull., No. 8.
C. Bazin, K. El-Ouassiti, and V. Ouellet, “Sequential leaching for the recovery of aluminum from a Canadian clay,” Hydrometallurgy, 88, 196–201 (2009), DOI: 10.1016/j.hydromet.2007.05.002.
J. Xiao, F. Li, Q. Zhong, et al., “Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC,” Hydrometallurgy, 155, 118–124 (2015), DOI: 10.1016/j.hydromet.2015.04.018.
P.-W. Zhu, H. Dai, L. Han, et al., “Aluminum extraction from coal ash by a two-step acid leaching method,” J. Zhejiang Univ.: Science A, 16, No. 2, 161–169 (2015), DOI: 10.1631/jzus.A1400195.
Y. Wu, L. Li, and M. Li, “Effect of pressure on alumina extraction from low-grade bauxite by acid-leaching method,” Light Metals’2014, pp. 121–123, DOI: 10.1002/9781118888438.ch21.
Ch.-y. Wu, H.-f. Yu, and H.-f. Zhang, “Extraction of aluminum by pressure acid-leaching method from coal fly ash,” Trans. Nonferr. Metals Soc. China, No. 22, 2282–2288 (2012), DOI: 10.1016/S1003-6326(11)61461-1.
B. Gillesberg, “Metal selection for severe corrosive applications,” Valve World, 16, No. 8, 79–83 (2011).
A. Robin and J. L. Rosa, “Corrosion behavior of niobium, tantalum and their alloys in hot hydrochloric and phosphoric acid solutions,” Int. J. Refract. Metals and Hard Mater., 18, No. 1, 13–21 (2000), DOI: 10.1016/S0263-4368(99)00034-7.
A. S. Senyuta, A. V. Panov, A. A. Damaskin, et al., “Study of filtration and washing of residue after HCL leaching of kaolin clay,” Light Metals’2015, pp. 127–130, DOI: 10.1002/9781119093435.ch23.
D. V. Valeev, Yu. A. Lainer, T. S. Vompe, and V. I. Pak, “Separation of chlorides of aluminum and iron by salting-out,” Izv. Samars. Nauch. Tsentra RAN, 16, No. 4(3), 512–515 (2014).
Y. Guo, X. Yang, H. Cui, et al., “Crystallization behavior of AlCl3–6H2O in hydrochloric system,” Huagong Xeubao/CIESC J., 65(10), 3960–3967 (2014), DOI: 10.3969/j.issn.0438-1157.2014.10.029.
G. P. Demopoulos, Z. Li, L. Becze, et al., “New technologies for HCL regeneration in chloride hydrometallurgy,” World of Metallurgy – ERZMETALL, 61, No. 2, 89–98 (2008).
L. Becze, S. J. Hock, and G. P. Demopoulos, “Precipitation of hematite and recovery of hydrochloric acid from concentrated iron chloride solutions by a novel hydrolytic decomposition process,” EPD Congress 2011, pp. 529–538, DOI: 10.1002/9781118495285.ch60.
D. V. Valeev, Yu. A. Lainer, A. B. Mikhailova, et al., “Decomposition of boehmite-kaolinite bauxites by hydrochloric acid with the use of preliminary roasting,” Perspekt. Mater., No. 4, 61–67 (2015).
D. V. Valeev, Yu. A. Lainer, and V. I. Pak, “Autoclave decomposition of boehmite-kaolinite bauxites with hydrochloric acid,” Perspekt. Mater., No. 7, 42–48 (2015).
A. G. Suss, A. A. Damaskin, A. S. Senyuta, et al., “Aspects of the behavior of different Al–Si minerals in the hydrochloric-acid decomposition of non-bauxite ores in Siberia,” Proc. 31st Int. Conf. ICSOBA, Krasnoyarsk, Russia, Sept. 3–6, 2013, pp. 433–438.
K. Liu, J. Xue, and W. Luo, “Effect of ultrasound on Al2O3 extraction rate during acid leaching process of coal fly ash,” Proc. EPD Congr. 2014 – TMS 2014 143rd Ann. Meeting and Exhibition, San Diego, U.S., Feb. 16–20, 2014, pp. 251–258.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Metallurg, No. 2, pp. 75–80, February, 2016.
Rights and permissions
About this article
Cite this article
Valeev, D.V., Lainer, Y.A., Mikhailova, A.B. et al. Reaction of Bauxite with Hydrochloric Acid Under Autoclave Conditions. Metallurgist 60, 204–211 (2016). https://doi.org/10.1007/s11015-016-0274-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-016-0274-y