Abstract
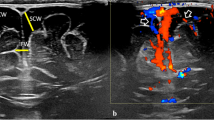
The first case of Glutaric aciduria Type 1(GA1) in an African child was reported in 2001. GA1 has a prevalence of 1:5000 in black South Africans. Although early diagnosis is essential for a favourable outcome, newborn screening is not routine in South Africa where an estimated 320,000 children have HIV infection. Neurodevelopmental delay and encephalopathy are complications of both HIV and GA1. In such a setting it is important to recognise that HIV and GA1 can occur simultaneously. We present an HIV-infected South African male child of Xhosa descent with macrocephaly who commenced combination antiretroviral therapy (ART) at 8 weeks of age in a clinical trial which included a neurodevelopmental sub-study. He developed short-lived focal seizures at 16 months after minor head trauma. Neurological examination was normal. Neuroimaging showed temporal lobe atrophy, subtle hyperintense signal change in the globus pallidus, and focal haemosiderosis in the right Sylvian fissure region. As findings were not in keeping with HIV encephalopathy, a urine metabolic screen was undertaken which suggested GA1. Genetic testing confirmed Arg293Trp mutation. He began L-carnitine and a low protein diet as a restricted diet was not practicable. At 21 months he developed pulmonary tuberculosis, requiring 6 months treatment. He did not develop any neurologic motor symptoms. Serial neurodevelopmental and neuropsychological test scores until 9 years were similar to healthy neighbourhood controls, except for mild language delay at 3½ years. Detection of GA1, probably facilitated through participation in a clinical trial, was pivotal for a favourable outcome. The concomitant use of ART and anti-tuberculous therapy in a child with GA1 appears safe.


Similar content being viewed by others
References
Baric I, Zschocke J, Christensen E, Duran M, Goodman SI, Leonard JV, Muller E, Morton DH, Superti-Furga A, Hoffmann GF (1998) Diagnosis and management of glutaric aciduria type I. J Inherit Metab Dis 21(4):326–340. https://doi.org/10.1023/A:1005390105171
Baric I, Wagner L, Feyh P, Liesert M, Buckel W, Hoffmann GF (1999) Sensitivity and specificity of free and total glutaric acid and 3-hydroxyglutaric acid measurements by stable-isotope dilution assays for the diagnosis of glutaric aciduria type I. J Inherit Metab Dis 22(8):867–881. https://doi.org/10.1023/A:1005683222187
Beery KE, Beery NA (2010) Beery VMI: The Beery-Buktenica developmental test of visual-motor integration. Administration, scoring and teaching manual. 6th ed, 6th edn. Pearson, San Antonio
Bjugstad KB, Goodman SI, Freed CR (2000) Age at symptom onset predicts severity of motor impairment and clinical outcome of glutaric acidemia type 1. J Pediatr 137(5):681–686. https://doi.org/10.1067/mpd.2000.108954
Boy N, Muhlhausen C, Maier EM, Heringer J, Assmann B, Burgard P, Dixon M, Fleissner S, Greenberg CR, Harting I, Hoffmann GF, Karall D, Koeller DM, Krawinkel MB, Okun JG, Opladen T, Posset R, Sahm K, Zschocke J, Kolker S, individual c A (2017) Proposed recommendations for diagnosing and managing individuals with glutaric aciduria type I: second revision. J Inherit Metab Dis 40(1):75–101. https://doi.org/10.1007/s10545-016-9999-9
Boyede G, Eley B, Donald K (2016) Preliminary Validation of a new developmental screening tool for neurodevelopmental delay in HIV-infected South African children. J Child Neurol 31(2):145–152. https://doi.org/10.1177/0883073815585351
Brinkman K, Smeitink JA, Romijn JA, Reiss P (1999) Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354(9184):1112–1115. https://doi.org/10.1016/S0140-6736(99)06102-4
Butorov EV (2013) Relationship between plasma L-lysine concentrations and levels of HIV-1 RNA. Virulence 4(7):646–653. https://doi.org/10.4161/viru.26361
Collins IJ, Judd A, Gibb DM (2014) Immediate antiretroviral therapy in young HIV-infected children: benefits and risks. Curr Opin HIV AIDS 9(1):87–94. https://doi.org/10.1097/COH.0000000000000027
Cornell M, Technau K, Fairall L, Wood R, Moultrie H, van Cutsem G, Giddy J, Mohapi L, Eley B, Mac Phail P, Prozesky H, Rabie H, Davies MA, Maxwell N, Boulle A, A. S. A. C. International epidemiologic Databases to Evaluate (2009) Monitoring the South African national antiretroviral treatment programme, 2003-2007: the IeDEA Southern Africa collaboration. S Afr Med J 99(9):653–660
Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, Josipovic D, Liberty A, Lazarus E, Innes S, van Rensburg AJ, Pelser W, Truter H, Madhi SA, Handelsman E, Jean-Philippe P, McIntyre JA, Gibb DM, Babiker AG, Team CS (2013) Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 382(9904):1555–1563. https://doi.org/10.1016/S0140-6736(13)61409-9
Crowell CS, Huo Y, Tassiopoulos K, Malee KM, Yogev R, Hazra R, Rutstein RM, Nichols SL, Smith RA, Williams PL, Oleske J, Muller WJ, Team PCS, H. C. S. the Pediatric (2015) Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS 29(3):295–304. https://doi.org/10.1097/QAD.0000000000000528
Drigo P, Piovan S, Battistella PA, Della Puppa A, Burlina AB (1996) Macrocephaly, subarachnoid fluid collection, and glutaric aciduria type I. J Child Neurol 11(5):414–417. https://doi.org/10.1177/088307389601100516
Faye A, Le Chenadec J, Dollfus C, Thuret I, Douard D, Firtion G, Lachassinne E, Levine M, Nicolas J, Monpoux F, Tricoire J, Rouzioux C, Tardieu M, Mayaux MJ, Blanche S (2004) Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis 39(11):1692–1698. https://doi.org/10.1086/425739
Goodman SI, Kohlhoff JG (1975) Glutaric aciduria: inherited deficiency of glutaryl-CoA dehydrogenase activity. Biochem Med 13(2):138–140
Goodman SI, Stein DE, Schlesinger S, Christensen E, Schwartz M, Greenberg CR, Elpeleg ON (1998) Glutaryl-CoA dehydrogenase mutations in glutaric acidemia (type I): review and report of thirty novel mutations. Hum Mutat 12(3):141–144. https://doi.org/10.1002/(SICI)1098-1004(1998)12:3<141::AID-HUMU1>3.0.CO;2-K
Gordon N (2006) Glutaric aciduria types I and II. Brain Dev 28(3):136–140. https://doi.org/10.1016/j.braindev.2005.06.010
Govender R, Mitha A, Mubaiwa L (2017) A review of patients with glutaric aciduria type 1 at Inkosi Albert Luthuli Central Hospital, Durban, South Africa. S Afr Med J 107(3):201–204. https://doi.org/10.7196/SAMJ.2017.v107i3.11332
Griffiths, R., 1996. The Griffiths mental development scales: from birth to 2 years. Revision by Huntley M. The Test Agency Ltd.
Harting I, Neumaier-Probst E, Seitz A, Maier EM, Assmann B, Baric I, Troncoso M, Muhlhausen C, Zschocke J, Boy NP, Hoffmann GF, Garbade SF, Kolker S (2009) Dynamic changes of striatal and extrastriatal abnormalities in glutaric aciduria type I. Brain 132(Pt 7):1764–1782. https://doi.org/10.1093/brain/awp112
Heringer J, Boy SP, Ensenauer R, Assmann B, Zschocke J, Harting I, Lucke T, Maier EM, Muhlhausen C, Haege G, Hoffmann GF, Burgard P, Kolker S (2010) Use of guidelines improves the neurological outcome in glutaric aciduria type I. Ann Neurol 68(5):743–752. https://doi.org/10.1002/ana.22095
Hoffmann GF, Athanassopoulos S, Burlina AB, Duran M, de Klerk JB, Lehnert W, Leonard JV, Monavari AA, Muller E, Muntau AC, Naughten ER, Plecko-Starting B, Superti-Furga A, Zschocke J, Christensen E (1996) Clinical course, early diagnosis, treatment, and prevention of disease in glutaryl-CoA dehydrogenase deficiency. Neuropediatrics 27(3):115–123. https://doi.org/10.1055/s-2007-973761
Kaufman AS, Kaufman NL (2004) Kaufman Assessment Battery for Children, 2nd edn. Pearson, Minneapolis
Kolker S, Garbade SF, Greenberg CR, Leonard JV, Saudubray JM, Ribes A, Kalkanoglu HS, Lund AM, Merinero B, Wajner M, Troncoso M, Williams M, Walter JH, Campistol J, Marti-Herrero M, Caswill M, Burlina AB, Lagler F, Maier EM, Schwahn B, Tokatli A, Dursun A, Coskun T, Chalmers RA, Koeller DM, Zschocke J, Christensen E, Burgard P, Hoffmann GF (2006) Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res 59(6):840–847. https://doi.org/10.1203/01.pdr.0000219387.79887.86
Kolker S, Sauer SW, Hoffmann GF, Muller I, Morath MA, Okun JG (2008) Pathogenesis of CNS involvement in disorders of amino and organic acid metabolism. J Inherit Metab Dis 31(2):194–204. https://doi.org/10.1007/s10545-008-0823-z
Kolker S, Christensen E, Leonard JV, Greenberg CR, Boneh A, Burlina AB, Burlina AP, Dixon M, Duran M, Garcia Cazorla A, Goodman SI, Koeller DM, Kyllerman M, Muhlhausen C, Muller E, Okun JG, Wilcken B, Hoffmann GF, Burgard P (2011) Diagnosis and management of glutaric aciduria type I--revised recommendations. J Inherit Metab Dis 34(3):677–694. https://doi.org/10.1007/s10545-011-9289-5
Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, van Rensburg AJ, Violari A, Babiker AG, Madhi SA, Jean-Philippe P, Gibb DM, Cotton MF (2012) Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 26(13):1685–1690. https://doi.org/10.1097/QAD.0b013e328355d0ce
Le Doare K, Bland R, Newell ML (2012) Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics 130(5):e1326–e1344. https://doi.org/10.1542/peds.2012-0405
Lindner M, Kolker S, Schulze A, Christensen E, Greenberg CR, Hoffmann GF (2004) Neonatal screening for glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis 27(6):851–859. https://doi.org/10.1023/B:BOLI.0000045769.96657.af
Luiz D, Faragher B, Barnard A, Knoesen N, Kotras N, Burns LE, Challis D (2006) Griffiths mental development scales - extended revised, two ot eight years. Hogrefe- The Test Agency ltd, Oxford
Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, Nachman S, Kayhty H, Klugman KP, Violari A, A. S. T. Comprehensive International Program of Research on (2010) Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis 202(3):355–361. https://doi.org/10.1086/653704
Nuss ET, Tanumihardjo SA (2010) Maize: a paramount staple crop in the context of global nutrition. Compr Rev Food Sci F 9(4):417–436. https://doi.org/10.1111/j.1541-4337.2010.00117.x
Ojwang PJ, Pegoraro RJ, Deppe WM, Sankar R, McKerrow N, Varughese L, Stoker AF, Goodman SI (2001) Biochemical and molecular diagnosis of glutaric aciduria type 1 in a black South African male child: case report. East Afr Med J 78(12):682–685
Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, Maartens G, McIlleron HM (2008) Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 47(5):566–569. https://doi.org/10.1097/QAI.0b013e3181642257
Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neuro-Oncol 18(5):388–399. https://doi.org/10.1007/s13365-012-0120-3
Silva MF, Aires CC, Luis PB, Ruiter JP, IJIst L, Duran M, Wanders RJ, Tavares de Almeida I (2008) Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis 31(2):205–216. https://doi.org/10.1007/s10545-008-0841-x
Strauss KA, Puffenberger EG, Robinson DL, Morton DH (2003) Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Med Genet C: Semin Med Genet 121C(1):38–52. https://doi.org/10.1002/ajmg.c.20007
Strauss KA, Brumbaugh J, Duffy A, Wardley B, Robinson D, Hendrickson C, Tortorelli S, Moser AB, Puffenberger EG, Rider NL, Morton DH (2011) Safety, efficacy and physiological actions of a lysine-free, arginine-rich formula to treat glutaryl-CoA dehydrogenase deficiency: focus on cerebral amino acid influx. Mol Genet Metab 104(1–2):93–106. https://doi.org/10.1016/j.ymgme.2011.07.003
Superti-Furga A, Hoffmann GF (1997) Glutaric aciduria type 1 (glutaryl-CoA-dehydrogenase deficiency): advances and unanswered questions. Report from an international meeting. Eur J Pediatr 156(11):821–828
Tiffin J (1968) Purdue pegboard: examiner manual. Science Research Associates, Chicago
Twomey EL, Naughten ER, Donoghue VB, Ryan S (2003) Neuroimaging findings in glutaric aciduria type 1. Pediatr Radiol 33(12):823–830. https://doi.org/10.1007/s00247-003-0956-z
van der Watt G, Owen EP, Berman P, Meldau S, Watermeyer N, Olpin SE, Manning NJ, Baumgarten I, Leisegang F, Henderson H (2010) Glutaric aciduria type 1 in South Africa-high incidence of glutaryl-CoA dehydrogenase deficiency in black South Africans. Mol Genet Metab 101(2–3):178–182. https://doi.org/10.1016/j.ymgme.2010.07.018
Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA, Team CS (2008) Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 359(21):2233–2244. https://doi.org/10.1056/NEJMoa0800971
Woelfle J, Kreft B, Emons D, Haverkamp F (1996) Subdural hemorrhage as an initial sign of glutaric aciduria type 1: a diagnostic pitfall. Pediatr Radiol 26(11):779–781
Zafeiriou DI, Zschocke J, Augoustidou-Savvopoulou P, Mauromatis I, Sewell A, Kontopoulos E, Katzos G, Hoffmann GF (2000) Atypical and variable clinical presentation of glutaric aciduria type I. Neuropediatrics 31(6):303–306. https://doi.org/10.1055/s-2000-12943
Zielonka M, Braun K, Bengel A, Seitz A, Kolker S, Boy N (2015) Severe Acute Subdural hemorrhage in a patient with glutaric aciduria type I after minor head trauma: a case report. J Child Neurol 30(8):1065–1069. https://doi.org/10.1177/0883073814541479
Zschocke J, Quak E, Guldberg P, Hoffmann GF (2000) Mutation analysis in glutaric aciduria type I. J Med Genet 37(3):177–181
Acknowledgements
We would like to thank the participant and his mother for taking part in the study and the KID-CRU personnel. The authors would like to thank Drs Jennifer Cartwright and George van der Watt for their contributions in managing this patient and advice on the manuscript. Support for the CHER trial was provided by the US National Institute of Allergy and Infectious Diseases through the CIPRA network, Grant U19 AI53217; the Departments of Health of the Western Cape and Gauteng, South Africa; and GlaxoSmithKline. Additional support was provided with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, United States Department of Health and Human Services, under Contract No. HHSN272200800014C. Neurodevelopmental assessments from 0 to 5 years were funded through grants from the Harry Crossley Foundation and the South African Medical Research Council, the National Research Foundation of South Africa and CIPRA-SA. Support for the CHER Plus follow on study, until 9 years was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant: R01HD071664-01 and ViiV Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The above child was enrolled on the neurodevelopmental sub-study of the CHER trial. The sub-study approved by the Stellenbosch University health research ethics committee (reg no N05/05/092 and M11/10/042) and was been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Consent to participate was obtained in person from the child’s mother in her home language. At 9 the child provided assent.
Rights and permissions
About this article
Cite this article
Thomas, A., Dobbels, E.F.M., Springer, P.E. et al. Favourable outcome in a child with symptomatic diagnosis of Glutaric aciduria type 1 despite vertical HIV infection and minor head trauma. Metab Brain Dis 33, 537–544 (2018). https://doi.org/10.1007/s11011-018-0196-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0196-4