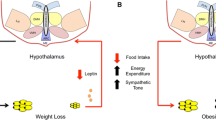
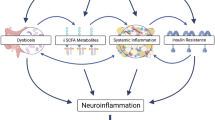
Abstract
Obesity has reached epidemic proportions in many countries around the world. Several studies have reported that obesity can lead to the development of cognitive decline. There is increasing evidence to demonstrate that microglia play a crucial role in cognitive decline in cases of obesity, Alzheimer’s disease and also in the aging process. Although there have been several studies into microglia over the past decades, the mechanistic link between microglia and cognitive decline in obese models is still not fully understood. In this review, the current available evidence from both in vitro and in vivo investigations regarding the association between the alteration in microglial activity in different obese models with respect to cognition are included. The metabolite profiles from obesity, adiposity, dietary and hormone affected microglial activation and its function in the brain are comprehensively summarized. In addition, the possible roles of microglial activation in relation to cognitive dysfunction are also presented and discussed. To ensure a balanced perspective controversial reports regarding these issues are included and discussed.


Similar content being viewed by others
References
Anderberg RH, Richard JE, Eerola K, López-Ferreras L, Banke E, Hansson C, Nissbrandt H, Berqquist F, Gribble FM, Reimann F, Wernstedt Asterholm I, Lamy CM, Skibicka KP (2017) Glucagon-like Peptide-1 and its analogues act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes 66(4):1062–1073. https://doi.org/10.2337/db16-0755
Andre C et al (2017) Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 66:908–919. https://doi.org/10.2337/db16-0586
Balland E, Cowley MA (2015) New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol 39:59–65. https://doi.org/10.1016/j.yfrne.2015.09.004
Bellavance MA, Rivest S (2014) The HPA - immune Axis and the Immunomodulatory actions of glucocorticoids in the brain. Front Immunol 5:136. https://doi.org/10.3389/fimmu.2014.00136
Bell-Temin H, Culver-Cochran AE, Chaput D, Carlson CM, Kuehl M, Burkhardt BR, Bickford PC, Liu B, Stevens SM Jr (2015) Novel molecular insights into classical and alternative activation states of microglia as revealed by stable isotope labeling by amino acids in cell culture (SILAC)-based proteomics. Mol. Cell. Proteomics 14:3173–3184. https://doi.org/10.1074/mcp.M115.053926
Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E (2015) Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci U S A 112(51):15731–15736. https://doi.org/10.1073/pnas.1511593112
Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin N Am 37:635–646, viii-ix. https://doi.org/10.1016/j.ecl.2008.06.007
Brocca ME, Pietranera L, Meyer M, Lima A, Roig P, de Kloet ER, De Nicola AF (2017) Mineralocorticoid receptor associates with pro-inflammatory bias in hippocampus of spontaneously hypertensive rats. J Neuroendocrinol 29(7). https://doi.org/10.1111/jne.12489
Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KLJ (2014) Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun 35:33–42. https://doi.org/10.1016/j.bbi.2013.06.007
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. https://doi.org/10.2337/db06-1491
Chhor V, le Charpentier T, Lebon S, Oré MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Sävman K, Mallard C, Gressens P, Fleiss B (2013) Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun 32:70–85. https://doi.org/10.1016/j.bbi.2013.02.005
Chunchai T, Samniang B, Sripetchwandee J, Pintana H, Pongkan W, Kumfu S, Shinlapawittayatorn K, KenKnight BH, Chattipakorn N, Chattipakorn SC (2016) Vagus nerve stimulation exerts the Neuroprotective effects in obese-insulin resistant rats, leading to the improvement of cognitive function. Sci Rep 6:26866. https://doi.org/10.1038/srep26866
Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC (2011) Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103:10–16. https://doi.org/10.1016/j.physbeh.2011.01.010
Collaboration NCDRF (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387:1377–1396. https://doi.org/10.1016/S0140-6736(16)30054-X
Davies DS, Ma J, Jegathees T, Goldsbury AC (2016) Microglia show altered morphology and reduced arborisation in human brain during aging and Alzheimer's disease. Brain Pathol 27(6):795–808. https://doi.org/10.1111/bpa.12456
Dey A, Hao S, Erion JR, Wosiski-Kuhn M, Stranahan AM (2014) Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J Neuroimmunol 269:20–27. https://doi.org/10.1016/j.jneuroim.2014.01.013
Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM (2011) Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 25:1113–1122. https://doi.org/10.1016/j.bbi.2011.02.008
Duffy CM, Xu H, Nixon JP, Bernlohr DA, Butterick TA (2017) Identification of a fatty acid binding protein4-UCP2 axis regulating microglial mediated neuroinflammation. Mol Cell Neurosci 80:52–57. https://doi.org/10.1016/j.mcn.2017.02.004
Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, Ghazavi H, Saberi Karimian M, Parizadeh SMR, Mohammadi M, Mohaddes Ardabili H, Ferns GA, Ghayour-Mobarhan M (2016) Association of Serum hs-CRP levels with the presence of obesity, diabetes mellitus, and other cardiovascular risk factors. J Clin Lab Anal 30:672–676. https://doi.org/10.1002/jcla.21920
Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM (2014) Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 34:2618–2631. https://doi.org/10.1523/JNEUROSCI.4200-13.2014
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK (2017) Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2(7):e91229. https://doi.org/10.1172/jci.insight.91229
Fernandes JT, Chutna O, Chu V, Conde JP, Outeiro TF (2016) A novel microfluidic cell co-culture platform for the study of the molecular mechanisms of Parkinson's disease and other Synucleinopathies. Front Neurosci 10:511. https://doi.org/10.3389/fnins.2016.00511
Franco R, Fernandez-Suarez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86. https://doi.org/10.1016/j.pneurobio.2015.05.003
Freeman LR, Small BJ, Bickford PC, Umphlet C, Granholm AC (2011) A high-fat/high-cholesterol diet inhibits growth of fetal hippocampal transplants via increased inflammation. Cell Transplant 20:1499–1514. https://doi.org/10.3727/096368910X557281
Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A (1987) Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol 17:1271–1278. https://doi.org/10.1002/eji.1830170909
Gao Y, Ottaway N, Schriever SC, Legutko B, García-Cáceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschöp MH, Yi CX (2014) Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62:17–25. https://doi.org/10.1002/glia.22580
Ginhoux F, Prinz M (2015) Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol 7:a020537. https://doi.org/10.1101/cshperspect.a020537
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Front Cell Neurosci 7:45. https://doi.org/10.3389/fncel.2013.00045
de Git KC, Adan RA (2015) Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev 16(3):207–224. https://doi.org/10.1111/obr.12243
Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL (2010) Changes in Melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151:1622–1632. https://doi.org/10.1210/en.2009-1019
Greenwood CE, Winocur G (2005) High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 26(Suppl 1):42–45. https://doi.org/10.1016/j.neurobiolaging.2005.08.017
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140–155. https://doi.org/10.1002/glia.10161
Hao S, Dey A, Yu X, Stranahan AM (2016) Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 51:230–239. https://doi.org/10.1016/j.bbi.2015.08.023
Hazama GI, Yasuhara O, Morita H, Aimi Y, Tooyama I, Kimura H (2005) Mouse brain IgG-like immunoreactivity: strain-specific occurrence in microglia and biochemical identification of IgG. J Comp Neurol 492:234–249. https://doi.org/10.1002/cne.20710
Hsuchou H, Kastin AJ, Pan W (2012) Blood-borne metabolic factors in obesity exacerbate injury-induced gliosis. J Mol Neurosci 47:267–277. https://doi.org/10.1007/s12031-012-9734-4
Hwang IK, Kim IY, Kim YN, Yi SS, Park IS, Min BH, Doo HK, Ahn SY, Kim YS, Lee IS, Yoon YS, Seong JK (2009) Comparative study on high fat diet-induced 4-hydroxy-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res 34:964–972. https://doi.org/10.1007/s11064-008-9846-y
Inoue K, Sakuma E, Morimoto H, Asai H, Koide Y, Leng T, Wada I, Xiong ZG, Ueki T (2016) Serum- and glucocorticoid-inducible kinases in microglia. Biochem Biophys Res Commun 478:53–59. https://doi.org/10.1016/j.bbrc.2016.07.094
Inoue T, Tanaka M, Masuda S, Ohue-Kitano R, Yamakage H, Muranaka K, Wada H, Kusakabe T, Shimatsu A, Hasegawa K, Satoh-Asahara N (2017) Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim Biophys Acta 1862(5):552–560. https://doi.org/10.1016/j.bbalip.2017.02.010
Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J (2006) Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res 55:352–360. https://doi.org/10.1016/j.neures.2006.04.008
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846. https://doi.org/10.1038/nature05482
Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjöholm Å (2012) GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation 9:276. https://doi.org/10.1186/1742-2094-9-276
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77:10–18. https://doi.org/10.1016/j.neuron.2012.12.023
Kim YJ, Hwang SY, Oh ES, Oh S, Han IO (2006) IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res 84:1037–1046. https://doi.org/10.1002/jnr.21011
Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7:e47713. https://doi.org/10.1371/journal.pone.0047713
Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, Yoon SY (2016) Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry 22(11):1576–1584. https://doi.org/10.1038/mp.2016.103
de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG (2013) Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33:4825–4833. https://doi.org/10.1523/JNEUROSCI.3806-12.2013
de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C (2014) Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav 136:31–38. https://doi.org/10.1016/j.physbeh.2014.01.016
Kvarta MD, Bradbrook KE, Dantrassy HM, Bailey AM, Thompson SM (2015) Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J Neurophysiol 114:1713–1724. https://doi.org/10.1152/jn.00359.2015
Kwon YH, Kim J, Kim CS, Tu TH, Kim MS, Suk K, Kim DH, Lee BJ, Choi HS, Park T, Choi MS, Goto T, Kawada T, Ha TY, Yu R (2017) Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett 591:1742–1751. https://doi.org/10.1002/1873-3468.12691
Lee JK, Chung J, Kannarkat GT, Tansey MG (2013) Critical role of regulator G-protein signaling 10 (RGS10) in modulating macrophage M1/M2 activation. PLoS One 8:e81785. https://doi.org/10.1371/journal.pone.0081785
Lee JJ, Wang PW, Yang IH, Huang HM, Chang CS, CL W, Chuang JH (2015) High-fat diet induces toll-like receptor 4-dependent macrophage/microglial cell activation and retinal impairment. Invest Ophthalmol Vis Sci 56:3041–3050. https://doi.org/10.1167/iovs.15-16504
Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, Andrews ZB (2015) A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology 156:1701–1713. https://doi.org/10.1210/en.2014-1961
Ma L, Allen M, Sakae N, Ertekin-Taner N, Graff-Radford NR, Dickson DW, Younkin SG, Sevlever D (2016) Expression and processing analyses of wild type and p.R47H TREM2 variant in Alzheimer's disease brains. Mol Neurodegener 11:72. https://doi.org/10.1186/s13024-016-0137-9
McEwen HJ, Inglis MA, Quennell JH, Grattan DR, Anderson GM (2016) Deletion of suppressor of cytokine signaling 3 from forebrain neurons delays infertility and onset of hypothalamic Leptin resistance in response to a high caloric diet. J Neurosci: Off J Soc Neurosci 36:7142–7153. https://doi.org/10.1523/JNEUROSCI.2714-14.2016
Moehle MS, West AB (2015) M1 and M2 immune activation in Parkinson's disease: foe and ally? Neuroscience 302:59–73. https://doi.org/10.1016/j.neuroscience.2014.11.018
Moller K et al (2016) Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukot Essent Fat Acids 106:39–49. https://doi.org/10.1016/j.plefa.2015.12.001
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Neniskyte U, Vilalta A, Brown GC (2014) Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. FEBS Lett 588:2952–2956. https://doi.org/10.1016/j.febslet.2014.05.046
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP (2016) Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64:300–316. https://doi.org/10.1002/glia.22930
Ouyang S, Hsuchou H, Kastin AJ, Wang Y, Yu C, Pan W (2014) Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. J Cereb Blood Flow Metab 34:43–51. https://doi.org/10.1038/jcbfm.2013.166
Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci 91:409–414. https://doi.org/10.1016/j.lfs.2012.08.017
Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338. https://doi.org/10.1210/en.2011-1502
Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2013) DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 37:839–849. https://doi.org/10.1111/ejn.12088
Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC (2011) Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci 88:619–627. https://doi.org/10.1016/j.lfs.2011.02.003
Pratchayasakul W, Sa-nguanmoo P, Sivasinprasasn S, Pintana H, Tawinvisan R, Sripetchwandee J, Kumfu S, Chattipakorn N, Chattipakorn SC (2015) Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm Behav 72:68–77. https://doi.org/10.1016/j.yhbeh.2015.04.023
Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG (2013) Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr) 35:1621–1636. https://doi.org/10.1007/s11357-012-9464-0
Samniang B, Shinlapawittayatorn K, Chunchai T, Pongkan W, Kumfu S, Chattipakorn SC, KenKnight BH, Chattipakorn N (2016) Vagus nerve stimulation improves cardiac function by preventing mitochondrial dysfunction in obese-insulin resistant rats. Sci Rep 6:19749. https://doi.org/10.1038/srep19749
Sa-Nguanmoo P et al (2016) FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav 85:86–95. https://doi.org/10.1016/j.yhbeh.2016.08.006
Sen T, Cawthon CR, Ihde BT, Hajnal A, DiLorenzo PM, de La Serre CB, Czaja K (2017) Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav 173:305–317. https://doi.org/10.1016/j.physbeh.2017.02.027
Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87:10–20. https://doi.org/10.1016/j.brainresbull.2011.10.004
Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2014) DPP-4 inhibitor and PPARgamma agonist restore the loss of CA1 dendritic spines in obese insulin-resistant rats. Arch Med Res 45:547–552. https://doi.org/10.1016/j.arcmed.2014.09.002
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33:256–266. https://doi.org/10.1002/1098-1136(200103)33:3<256::AID-GLIA1024>3.0.CO;2-J
Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PCN, Joosten LAB, Netea MG, Kanneganti TD (2011) Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108:15324–15329. https://doi.org/10.1073/pnas.1100255108
Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP (2008) Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18:1085–1088. https://doi.org/10.1002/hipo.20470
Stranahan AM, Hao S, Dey A, Yu X, Baban B (2016) Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab 36:2108–2121. https://doi.org/10.1177/0271678X16642233
Su F, Yi H, Xu L, Zhang Z (2015) Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 294:60–68. https://doi.org/10.1016/j.neuroscience.2015.02.028
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201:647–657. https://doi.org/10.1084/jem.20041611
Tamargo IA, Bader M, Li Y, Yu SJ, Wang Y, Talbot K, DiMarchi RD, Pick CG, Greig NH (2016) Novel GLP-1R/GIPR co-agonist "twincretin" is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp Neurol:176–186. https://doi.org/10.1016/j.expneurol.2016.11.005
Tang Y, Le W (2015) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Tentillier N, Etzerodt A, Olesen MN, Rizalar FS, Jacobsen J, Bender D, Moestrup SK, Romero-Ramos M (2016) Anti-inflammatory modulation of microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson's disease model. J Neurosci Off J Soc Neurosci 36:9375–9390. https://doi.org/10.1523/JNEUROSCI.1636-16.2016
Oh-I S, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW (2010) Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. AJP: Endocrinol Metab 299(1):E47–E53. https://doi.org/10.1152/ajpendo.00026.2010
Tracy LM, Bergqvist F, Ivanova EV, Jacobsen KT, Iverfeldt K (2013) Exposure to the saturated free fatty acid Palmitate alters BV-2 microglia inflammatory response. J Mol Neurosci 51:805–812. https://doi.org/10.1007/s12031-013-0068-7
Trang T, Beggs S, Salter MW (2011) Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol 7:99–108. https://doi.org/10.1017/S1740925X12000087
Tsunekawa T, Banno R, Mizoguchi A, Sugiyama M, Tominaga T, Onoue T, Hagiwara D, Ito Y, Iwama S, Goto M, Suga H, Sugimura Y, Arima H (2017) Deficiency of PTP1B attenuates hypothalamic inflammation via activation of the JAK2-STAT3 pathway in microglia. EBioMedicine 16:172–183. https://doi.org/10.1016/j.ebiom.2017.01.007
Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16:543–551. https://doi.org/10.1038/nn.3358
Vaughn AC, Cooper EM, DiLorenzo PM, O'Loughlin LJ, Konkel ME, Peters JH, Hajnal A, Sen T, Lee SH, de la Serre CB, Czaja K (2017) Energy-dense diet triggers changes in gut microbiota, reorganization of gutbrain vagal communication and increases body fat accumulation. Acta Neurobiol Exp (Wars) 77:18–30
Wake H, Moorhouse AJ, Miyamoto A, Nabekura J (2013) Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci 36:209–217. https://doi.org/10.1016/j.tins.2012.11.007
Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A (2012) Saturated fatty acids activate microglia via toll-like receptor 4/NF-kappaB signalling. Br J Nutr 107:229–241. https://doi.org/10.1017/S0007114511002868
Wang WY, Tan MS, JT Y, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med 3:136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Wee YS, Weis JJ, Gahring LC, Rogers SW, Weis JH (2015) Age-related onset of obesity corresponds with metabolic Dysregulation and altered microglia morphology in mice deficient for Ifitm proteins. PLoS One 10:e0123218. https://doi.org/10.1371/journal.pone.0123218
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG (2011) B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17:610–617. https://doi.org/10.1038/nm.2353
Yang J, Kim CS, Tu T, Kim MS, Goto T, Kawada T, Choi MS, Park T, Sung MK, Yun J, Choe SY, Lee J, Joe Y, Choi HS, Back S, Chung H, Yu R (2017) Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients 9. https://doi.org/10.3390/nu9070650
Yi C-X, al-Massadi O, Donelan E, Lehti M, Weber J, Ress C, Trivedi C, Müller TD, Woods SC, Hofmann SM (2012a) Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav 106:485–490. https://doi.org/10.1016/j.physbeh.2012.03.021
Yi CX, Tschop MH, Woods SC, Hofmann SM (2012b) High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis Model Mech 5:686–690. https://doi.org/10.1242/dmm.009464
Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z (2016) Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation 13:77. https://doi.org/10.1186/s12974-016-0541-7
Zhang QS, Heng Y, Yuan YH, Chen NH (2016) Pathological alpha-synuclein exacerbates the progression of Parkinson's disease through microglial activation. Toxicol Lett 265:30–37. https://doi.org/10.1016/j.toxlet.2016.11.002
Zhu C, Xu B, Sun X, Zhu Q, Sui Y (2016) Targeting CCR3 to reduce amyloid-beta production, tau hyperphosphorylation, and synaptic loss in a mouse model of Alzheimer's disease. Mol Neurobiol 54(10):7964–7978. https://doi.org/10.1007/s12035-016-0269-5
Zhuang P, Shou Q, Lu Y, Wang G, Qiu J, Wang J, He L, Chen J, Jiao J, Zhang Y (2017) Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim Biophys Acta 1863:2715–2726. https://doi.org/10.1016/j.bbadis.2017.07.003
Acknowledgements
The authors thank Ms. Maria Love for her editorial assistance of this manuscript.
This work was supported by the Thailand Research Fund (RTA 6080003: SCC); the Royal Golden Jubilee PhD program (PHD/0146/2558 TC&SC); a NSTDA Research Chair Grant from the National Science and Technology Development Agency Thailand (NC) and a Chiang Mai University Center of Excellence Award (NC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chunchai, T., Chattipakorn, N. & Chattipakorn, S.C. The possible factors affecting microglial activation in cases of obesity with cognitive dysfunction. Metab Brain Dis 33, 615–635 (2018). https://doi.org/10.1007/s11011-017-0151-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0151-9