Abstract
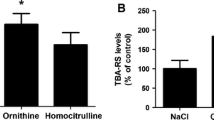
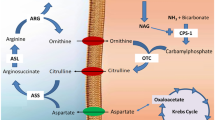
Ornithine, ammonia and homocitrulline are the major metabolites accumulating in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome, a genetic disorder characterized by neurological regression whose pathogenesis is still not understood. The present work investigated the in vivo effects of intracerebroventricular administration of ornithine and homocitrulline in the presence or absence of hyperammonemia induced by intraperitoneal urease treatment on a large spectrum of oxidative stress parameters in cerebral cortex from young rats in order to better understand the role of these metabolites on brain damage. Ornithine increased thiobarbituric acid-reactive substances (TBA-RS) levels and carbonyl formation and decreased total antioxidant status (TAS) levels. We also observed that the combination of hyperammonemia with ornithine resulted in significant decreases of sulfhydryl levels, reduced glutathione (GSH) concentrations and the activities of catalase (CAT) and glutathione peroxidase (GPx), highlighting a synergistic effect of ornithine and ammonia. Furthermore, homocitrulline caused increases of TBA-RS values and carbonyl formation, as well as decreases of GSH concentrations and GPx activity. Hcit with hyperammonemia (urease treatment) decreased TAS and CAT activity. We also showed that urease treatment per se was able to enhance TBA-RS levels. Finally, nitric oxide production was not altered by Orn and Hcit alone or in combination with hyperammonemia. Our data indicate that the major metabolites accumulating in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome provoke lipid and protein oxidative damage and a reduction of the antioxidant defenses in the brain. Therefore, it is presumed that oxidative stress may represent a relevant pathomechanism involved in the brain damage found in patients affected by this disease.






Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Change in thiol content and expression of glutathione redox system gene in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. doi:10.1016/S0304-3940(01)01636-6
Al-Hassnan ZN, Rashed MS, Al-Dirbashi OY, Patay Z, Rahbeeni Z, Abu-Amero KK (2008) Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome with stroke-like imaging presentation: clinical, biochemical and molecular analysis. J Neurol Sci 15(264):187–194. doi:10.1016/j.jns.2007.08.003
Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Zanatta A, Viegas CM, Dutra-Filho CS, Wajner M (2009) Evidence that the major metabolites accumulating in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome induce oxidative stress in brain of young rats. Int J Dev Neurosci 27:635–641. doi:10.1016/j.ijdevneu.2009.08.004
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT (2009) Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 29(6):783–788. doi:10.1111/j.1478-3231.2009.02034.x
Camacho JA, Obie C, Biery B, Goodman BK, Hu CA, Almashanu S, Steel G, Casey R, Lambert M, Mitchell GA, Valle D (1999) Hyperornithinaemia-hyperammonaemia-homocitrullinuria syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat Genet 22:151–158. doi:10.1038/9658
Cooper AJ, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. doi:10.1016/S0009-8981(03)00003-2
Diemer NH, Laursen H (1977) Glial cell reactions in rats with hyperammoniemia induced by urease or porto-caval anastomosis. Acta Neurol Scand 55(6):425–442
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi E (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266. doi:10.1006/abbi.2001.2292
Halliwell B, Gutteridge JMC (1996) Oxygen radicals and nervous system. Trends Neurosci 8:22–26
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. In: Halliwell B, Gutteridge JMC (eds) Free Radicals in Biology and Medicine. Oxford University Press, Oxford, pp 268–340
Haust MD, Gatfield PD, Gordon BA (1981) Ultrastructure of hepatic mitochondria in a child with hyperornithinemia, hyperammonemia, and homocitrullinuria. Hum Pathol 12:212–222
Hoffmann GF, Meier-Augenstein W, Stöckler S, Surtees R, Rating D, Nyhan WL (1993) Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis 16(4):648–669
Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231:30–39
Korman SH, Kanazawa N, Abu-Libdeh B, Gutman A, Tsujino S (2004) Hyperornithinemia, hyperammonemia, and homocitrullinuria syndrome with evidence of mitochondrial dysfunction due to a novel SLC25A15 (ORNT1) gene mutation in a Palestinian family. J Neurol Sci 218:53–58. doi:10.1016/j.jns.2003.10.017
Kosenko E, Kaminsky Y, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V (1997) Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res 27(6):637–644
Kosenko E, Venediktova N, Kaminsky Y, Montoliu C, Felipo V (2003) Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res 981(1–2):193–200. doi:10.1016/S0006-8993(03)03035-X
Kuhn DM, Aretha CW, Geddes TJ (1999) Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci 19:10289–10294
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marklund SL (1985) Pyrogallol autoxidation. In: Greenwald RA (ed) Handbook for Oxygen Radical Research, 1st edn. CRC Press, Boca Raton, FL, pp 243–247
Martinez-Hernandez A, Bell KP, Norenberg MD (1977) Glutamine synthetase: Glial localization in brain. Science 195:1356–1358
Metoki K, Hommes FA, Dyken P, Kelloes C, Trefz J (1984) Ultrastructural changes in fibroblast mitochondria of a patient with HHH-syndrome. J Inherit Metab Dis 7:147–150
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84:407–412
Murthy CR, Rama Rao KV, Bai G, Norenberg MD (2001) Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res 66(2):282–288. doi:10.1002/jnr.1222
Navarro-Gonzálvez JA, García-Benayas C, Arenas J (1998) Semiautomated measurement of nitrate in biological fluids. Clin Chem 44(3):679–681
NIH publication number 85–23 (1996), Revised guide for the care and use of laboratory animals NIH guide volume 25, number 28
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press
Reinehr R, Görg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Häussinger D (2007) Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 55(7):758–771. doi:10.1002/glia.20504
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Salvi S, Santorelli FM, Bertini E, Boldrini R, Meli C, Donati A, Burlina AB, Rizzo C, Di Capua M, Fariello G, Dionisi-Vici C (2001) Clinical and molecular findings in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Neurology 57:911–914
Schultz V, Lowenstein JM (1978) The purine nucleotide cycle. Studies of ammonia production and interconversions of adenine and hypoxanthine nucleotides and nucleosides by rat brain in situ. J Biol Chem 253:1938–1943
Singh P, Jain A, Kaur G (2004) Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 260(1–2):153–159
Tessa A, Fiermonte G, Dionisi-Vici C, Paradies E, Baumgartner MR, Chien YH, Loguercio C, de Baulny HO, Nassogne MC, Schiff M, Deodato F, Parenti G, Rutledge SL, Vilaseca MA, Melone MA, Scarano G, Aldamiz-Echevarría L, Besley G, Walter J, Martinez-Hernandez E, Hernandez JM, Pierri CL, Palmieri F, Santorelli FM (2009) Identification of novel mutations in the SLC25A15 gene in hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome: a clinical, molecular, and functional study. Hum Mutat 30(5):741–748. doi:10.1002/humu.20930
Valle D, Simell O (2001) The hyperornithinemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and Molecular Basis of Inherited Diseases, 8th edn. McGraw-Hill, New York, pp 1857–1896
Viegas CM, Zanatta A, Knebel LA, Schuck PF, Tonin AM, Ferreira Gda C, Amaral AU, Dutra Filho CS, Wannmacher CM, Wajner M (2009) Experimental evidence that ornithine and homocitrulline disrupt energy metabolism in brain of young rats. Brain Res 1291:102–112. doi:10.1016/j.brainres.2009.07.021
Viegas CM, Busanello EN, Tonin AM, de Moura AP, Grings M, Ritter L, Schuck PF, Ferreira Gda C, Sitta A, Vargas CR, Wajner M (2011) Dual mechanism of brain damage induced in vivo by the major metabolites accumulating in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Brain Res 19(1369):235–244. doi:10.1016/j.brainres.2010.10.112
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–332
Yagi K (1998) Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 108:107–110
Yu TW, Ong CN (1999) Lag-time measurement of antioxidant capacity using myoglobin and 2,2′-azino-bis(3-ethyl-benzthiazoline-6-sulfonic acid): rationale, application and limitation. Anal Biochem 275:217–223
Acknowledgments
We are grateful to the financial support of CNPq, PROPESq/UFRGS, FAPERGS, PRONEX, FINEP Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00 and Instituto Nacional de Ciência e Tecnologia- Neurotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viegas, C.M., Tonin, A.M., Zanatta, Â. et al. Impairment of brain redox homeostasis caused by the major metabolites accumulating in hyperornithinemia–hyperammonemia–homocitrullinuria syndrome in vivo. Metab Brain Dis 27, 521–530 (2012). https://doi.org/10.1007/s11011-012-9327-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9327-5