Abstract
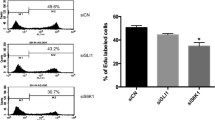
Abnormal activity of ERK/MAPK and PI3K/AKT pathways is one of the most important factors for the development of many cancer types including neuroblastoma cancer. Apart from these two pathways, some cell cycle regulators such as Speedy/RINGO also contribute to neuroblastoma development. There is data reinforcing the possible communication of the components of ERK/MAPK and PI3K/AKT pathways in carcinogenic process. In addition to this, there are studies about the direct/indirect interaction of Speedy/RINGO with these pathways in different cell types other than neuroblastoma. However, there is not any study available showing the interaction of Speedy/RINGO with both pathways in neuroblastoma cells. Therefore, the aim of this study is to determine the possible effect of Speedy/RINGO on PI3K/AKT and ERK/MAPK pathways in SH-SY5Y neuroblastoma cells. For this aim, Speedy/RINGO was silenced by siRNA technique to analyze the effects of direct inhibition of Speedy/RINGO on these pathways. Results showed that Speedy/RINGO silencing caused a significant decrease in MEK1/2 expression and AKT phosphorylation. Afterward, MEK1/2 was inhibited using a specific inhibitor U0126. Data reveal a corresponding decrease in the Speedy/RINGO expression and AKT phosphorylation indicating a reciprocal interaction between ERK/MAPK and Speedy/RINGO. In addition, MTS analysis showed that both ERK/MAPK inhibition and Speedy/RINGO silencing significantly reduced the viability of SH-SY5Y cells. This study provides information about a possible interaction of Speedy/RINGO with PI3K/AKT and ERK/MAPK pathways in SH-SY5Y cells for the first time. It will not only help to better understand the cancer-prone interactions of these pathways but also enable us to identify the appropriate molecular targets for developing efficient treatment strategies.




Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
In this study, no content that will require code availability.
References
Park JR, Eggert A, Caron H (2010) Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am 24(1):65–86. https://doi.org/10.1016/j.hoc.2009.11.011
Kaneko Y, Kanda N, Maseki N, Sakurai M, Tsuchida Y, Takeda T, Okabe I, Sakurai M (1987) Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res 47(1):311–318
Tanaka T, Slamon DJ, Shimoda H, Waki C, Kawaguchi Y, Tanaka Y, Ida N (1988) Expression of Ha-ras oncogene products in human neuroblastomas and the significant correlation with a patient’s prognosis. Cancer Res 48(4):1030–1034
Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard M et al (2015) Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 47:864–871. https://doi.org/10.1038/ng.3333
Johnsen JI, Segerström L, Orrego A, Elfman L, Henriksson M, Kågedal B, Eksborg S, Sveinbjörnsson B, Kogner P (2008) Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene 27:2910–2922. https://doi.org/10.1038/sj.onc.1210938
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838–2849. https://doi.org/10.1038/sj.onc.1207556
Xu N, Lao Y, Zhang Y, Gillespie DA (2012) Akt: a double-edged sword in cell proliferation and genome stability. J Oncol 2012:15. https://doi.org/10.1155/2012/951724
Porta C, Paglino C, Mosca A (2014) Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol 4:64. https://doi.org/10.3389/fonc.2014.00064
Opel D, Poremba C, Simon T, Debatin KM, Fulda S (2007) Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res 67(2):735–745. https://doi.org/10.1158/0008-5472.CAN-06-2201
Li Z, Thiele CJ (2007) Targeting Akt to increase the sensitivity of neuroblastoma to chemotherapy: lessons learned from the brainn-derived neurotrophic factor/TrkB signal transduction pathway. Expert Opin Ther Targets 11:1611–1621. https://doi.org/10.1517/14728222.11.12.1611
Boller D, Schramm A, Doepfner KT, Shalaby T, Von Bueren AO, Eggert A, Grotzer MA, Arcaro A (2008) Targeting the phosphoinositide 3-kinase isoform p110δ impairs growth and survival in neuroblastoma cells. Clin Cancer Res 14(4):1172–1181. https://doi.org/10.1158/1078-0432.CCR-07-0737
Paz-Ares L, Blanco-Aparicio C, García-Carbonero R, Carnero A (2009) Inhibiting PI3K as a therapeutic strategy against cancer. Clin Transl Oncol 11(9):572–579. https://doi.org/10.1007/s12094-009-0407-x
Kolch W (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351:289–305. https://doi.org/10.1042/0264-6021:3510289
Simon C, Hicks MJ, Nemechek AJ, Mehta R, O’Malley BW, Goepfert H, Flaitz CM, Boyd D (1999) PD 098059, an inhibitor of ERK1 activation, attenuates the in vivo invasiveness of head and neck squamous cell carcinoma. Br J Cancer 80(9):1412–1419. https://doi.org/10.1038/sj.bjc.6690537
Mattingly RR, Milstein ML, Mirkin BL (2001) Down-regulation of growth factor-stimulated MAP kinase signaling in cytotoxic drug-resistant human neuroblastoma cells. Cell Signal 13(7):499–505. https://doi.org/10.1016/s0898-6568(01)00173-5
Aksamitiene E, Kiyatkin A, Kholodenko BN (2012) Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Tran 40(1):139–146. https://doi.org/10.1042/BST20110609
Lubanska D, Porter LA (2014) The atypical cell cycle regulator Spy1 suppresses differentiation of the neuroblastoma stem cell population. Oncoscience 1(5):336–348. https://doi.org/10.18632/oncoscience.36
Chauhan S, Zheng X, Tan YY, Tay BH, Lim S, Venkatesh B, Kaldis P (2012) Evolution of the Cdk-activator Speedy/RINGO in vertebrates. Cell Mol Life Sci 69(22):3835–3850. https://doi.org/10.1007/s00018-012-1050-1
Matsuo T, Seth P, Thiele CJ (2001) Increased expression of p27Kip1 arrests neuroblastoma cell growth. Med Pediatr Oncol 36(1):97–99. https://doi.org/10.1002/1096-911X(20010101)36:1<97:AID-MPO1022>3.0.CO;2-X
Molenaar JJ, Ebus ME, Geerts D, Koster J, Lamers F, Valentijn LJ, Westerhout EM, Versteeg R, Caron HN (2009) Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells. Proc Nati Acad Sci USA 106(31):12968–12973. https://doi.org/10.1073/pnas.0901418106
Golipour A, Myers D, Seagroves T, Murphy D, Evan GI, Donoghue DJ, Moorehead RA, Porter LA (2008) The Spy1/RINGO family represents a novel mechanism regulating mammary growth and tumorigenesis. Cancer Res 68(10):3591–3600. https://doi.org/10.1158/0008-5472.CAN-07-6453
Liu ML, Cheng YM, Jia MC (2010) LM23 is essential for spermatogenesis in Rattus norvegicus. Front Biosci (Elite Ed) 1(2):187–194. https://doi.org/10.2741/e81
Pengda L, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W (2014) Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 508(7497):541–545. https://doi.org/10.1038/nature13079
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
McAndrew CW, Gastwirt RF, Donoghue DJ (2009) The atypical CDK activator Spy1 regulates the intrinsic DNA damage response and is dependent upon p53 to inhibit apoptosis. Cell Cycle 8(1):66–75. https://doi.org/10.4161/cc.8.1.7451
Porter LA, Dellinger RW, Tynan JA, Barnes EA, Kong M, Lenormand JL, Donoghue DJ (2002) Human Speedy: a novel cell cycle regulator that enhances proliferation through activation of Cdk2. J Cell Biol 157(3):357–366. https://doi.org/10.1083/jcb.200109045
Morris MC, Gondeau C, Tainer JA, Divita G (2002) Kinetic mechanism of activation of the Cdk2/cyclin A complex: key role of the C-lobe of the Cdk. J Biol Chem 277(26):23847–23853. https://doi.org/10.1074/jbc.M107890200
Acknowledgements
This study was supported by grant to Aysegul Yildiz from Mugla Sitki Kocman University Scientific Research Project Office, Research and Development Projects (Project Numbers: 17/251 and 17/023). We sincerely thank Prof. Dr. Uygar Halis Tazebay from Gebze Technical University, Department of Molecular Biology and Genetics and Prof. Dr. Arzu Karabay Korkmaz from lstanbul Technical University, Faculty of Science and Letters, Molecular Biology and Genetics Department for allowing us to use their laboratory infrastructure. We would also thank Assoc. Prof. Emin Ilker Medine from Ege University Institute of Nuclear Sciences for his help about providing SH-SY5Y neuroblastoma cell line.
Funding
This study was funded by grants from the Scientific Research Project Office of Mugla Sitki Kocman University (Project Numbers: 17/251 and 17/023).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [AY], [YK] and [SK]. The first draft of the manuscript was written by [AY] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they there are no competing interests.
Ethical approval
Ethic approval is not required for this study.
Consent to participate
In this study, no content that will require consent to participate.
Consent for publication
In this study, no content that will require consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaya, Y., Kucukvardar, S. & Yildiz, A. Speedy/RINGO protein interacts with ERK/MAPK and PI3K/AKT pathways in SH-SY5Y neuroblastoma cells. Mol Cell Biochem 473, 133–141 (2020). https://doi.org/10.1007/s11010-020-03813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03813-8