Abstract
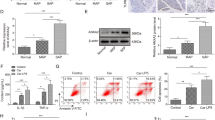
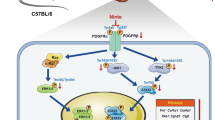
Acute pancreatitis (AP) is an inflammatory disease caused by the abnormal activation of pancreatic enzymes in the pancreas, with a considerably high morbidity and mortality. However, the etiological factor and pathogenesis of AP are still unclear. This study was aimed to explore the role and mechanism of interferon regulatory factor 9 (IRF9) in the occurrence of AP and to provide experimental and theoretical foundation for AP diagnosis and treatment. AP model in vitro was established by caerulein-induced group. Small interfering RNA (siRNA) was designed and constructed to silence IRF9 gene. After siRNA transfected and caerulein treated successfully, the expression levels of IRF9, SIRT1, and acetylated p53 (Ac-p53) were determined by qRT-PCR and Western blot. The apoptosis, proliferation, and migration of AR42J cells were checked by flow cytometry, MTT, and transwell assay. Dual-luciferase reporter assay was implemented to validate the regulatory effect of IRF9 on SIRT1. Here, our study showed that the expression of IRF9 and Ac-p53 was increased, SIRT1 was decreased, and cell apoptosis, proliferation, and migration of AR42J cells were increased after caerulein induced. IRF9 gene silencing upregulated SIRT1, downregulated Ac-p53, and inhibited cell apoptosis, proliferation, and migration. Dual-Luciferase reporter assay showed that IRF9 could negatively regulate SIRT1. The potential mechanism was that IRF9 could modulate cell apoptosis, proliferation, migration, and bind the promoter of SIRT1 to repress SIRT1-p53. It hinted that IRF9 showed a novel function in AP by modulating cell apoptosis, proliferation, migration, and suppressing SIRT1-p53. IRF9 might be a good potential treatment target for AP.







Similar content being viewed by others
References
Johnson CD, Besselink MG, Carter R (2014) Acute pancreatitis. BMJ 349:g4859. https://doi.org/10.1136/bmj.g4859
Wu BU, Banks PA (2013) Clinical management of patients with acute pancreatitis. Gastroenterology 144:127–1281. https://doi.org/10.1053/j.gastro.2013.01.075
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS (2013) Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitionsby international consensus. Gut 62:102–111. https://doi.org/10.1136/gutjnl-2012-302779
Frossard JL, Steer ML, Pastor CM (2008) Acute pancreatitis. Lancet 371:143–152. https://doi.org/10.1016/S0140-6736(08)60107-5
Bollen TL (2016) Acute pancreatitis: international classification and nomenclature. Clin Radiol 71:121–133. https://doi.org/10.1016/j.crad.2015.09.013
Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG (2019) Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastrenterology 156:1994–2007. https://doi.org/10.1053/j.gastro.2019.01.269
Koizumi M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Takeda K, Isaji S, Otsuki M, Matsuno S (2006) JPN Guidelines for the management of acute pancreatitis: diagnostic criteria for acute pancreatitis. J Hepatobiliary Surg 13:25–32. https://doi.org/10.1007/s00534-005-1048-2
Forsmark CE, Swaroop VS, Wilcox CM (2016) Acute pancreatitis. N Engl J Med 375:1972–1981. https://doi.org/10.1056/NEJMra1505202
Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, Dibonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ (2012) Burden of gastrointestinal disease in the United States. Gastroenterology 143:1179–1187. https://doi.org/10.1053/j.gastro.2012.08.002
Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, Dennison AR (2009) Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology 9:601–614. https://doi.org/10.1159/000212097Epub 2009
Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, Gurusamy KS (2017) Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev 4:CD011384. https://doi.org/10.1002/14651858
Sun JK, Li WQ, Ke L, Tong ZH, Ni HB, Li G, Zhang LY, Nie Y, Wang XY, Ye XH, Li N, Li JS (2013) Early enteral nutrition prevents intra-abdominal hypertension and reduces the severity of severe acute pancreatitis compared with delayed enteral nutrition: a prospective pilot study. World J Surg 37:2053–2060. https://doi.org/10.1007/s00268-013-2087-5
Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY (2013) Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol 19:917–922. https://doi.org/10.3748/wjg.v19.i6.917
Chen Y, Ma L, Song X, Fei J, Chen E, Mao E (2015) Beneficial effects of fluid resuscitation via the rectum on hemodynamic disorders and multiple organ injuries in an experimental severe acute pancreatitis model. Pancreatology 15:626–634. https://doi.org/10.1016/j.pan.2015.09.001
Furey C, Buxbaum J, Chambliss AB (2020) A review of biomarker utiliation in the diagnosis and management of acute pancreatitis reveals amylase ordering is favored in patients requiring laparoscopic cholecystectomy. Clin Biochem 77:54–56. https://doi.org/10.1016/j.clinbiochem.2019.12.014
Matta B, Song S, Li D, Barnes BJ (2017) Interferon regulatory factor signaling in autoimmune disease. Cytokine 98:15–26. https://doi.org/10.1016/j.cyto.2017.02.006
Marsili G, Perrotti E, Remoli AL, Acchioni C, Sgarbanti M, Battistini A (2016) IFN regulatory factors and antiviral innate immunity: how viruses can get better. J Interferon Cytokine Res 36:414–432. https://doi.org/10.1089/jir.2016.0002
Abrams SI, Netherby CS, Twum DY, Messmer MN (2016) Relevance of interferon regulatory factor-8 expression in myeloid-tumor interactions. J Interferon Cytokine Res 36:442–453. https://doi.org/10.1089/jir.2015.0174
Chen YJ, Li J, Lu N, Shen XZ (2017) Interferon regulatory factors: a key to tumour immunity. Int Immunopharmacol 49:1–5. https://doi.org/10.1016/j.intimp.2017.05.010
Zhang Y, Li H (2017) Reprogramming interferon regulatory factor signaling in cardiometabolic diseases. Physiology (Bethesda) 32:210–223. https://doi.org/10.1152/physiol.00038.2016
Alsamman K, El-Masry OS (2018) Interferon regulatory factor 1 inactivation in human cancer. Biosci Rep 38:1672
Chen HH, Stewart AFR (2017) Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery. Neural Regen Res 12:1762–1764. https://doi.org/10.4103/1673-5374
Wang PX, Zhang R, Huang L, Zhu LH, Jiang DS, Chen HZ, Zhang Y, Tian S, Zhang XF, Zhang XD, Liu DP, Li H (2014) Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. J Hepatol 62:111–120. https://doi.org/10.1016/j.jhep.2014.08.022
Zhang SM, Zhu LH, Chen HZ, Zhang R, Zhang P, Jiang DS, Gao L, Tian S, Wang L, Zhang Y, Wang PX, Zhang XF, Zhang XD, Liu DP, Li H (2014) Interferon regulatory factor 9 is critical for neointima formation following vascular injury. Nat Commun 5:5160. https://doi.org/10.1038/ncomms6160
Wan W, Ding Y, Xie Z, Li Q, Yan F, Budbazar E, Pearce WJ, Hartman R, Obenaus A, Zhang JH, Jiang Y, Tang J (2019) PDGFR-β modulates vascular smooth muscle cell phenotype via IRF-9/SIRT-1/NF-κB pathway in subarachnoid hemorrhage rats. J Cereb Blood Flow Metab 39:1369–1380. https://doi.org/10.1177/0271678X18760954
Chen HZ, Guo S, Li ZZ, Lu Y, Jiang DS, Zhang R, Lei H, Gao L, Zhang X, Zhang Y, Wang L, Zhu LH, Xiang M, Zhou Y, Wan Q, Dong H, Liu DP, Li H (2014) A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J Neurosci 34:11897–11912. https://doi.org/10.1523/Jneurosci.1545-14.2014
Zhang Y, Liu X, She ZG, Jiang DS, Wan N, Xia H, Zhu XH, Wei X, Zhang XD, Li H (2014) Interferon regulatory factor 9 is an essential mediator of heart dysfunction and cell death following myocardial ischemia/reperfusion injury. Basic Res Cardiol 109:434. https://doi.org/10.1007/s00395-014-0434-9
Tian WL, Guo R, Wang F, Jiang ZX, Tang P, Huang YM, Sun L (2018) The IRF9-SIRT1-P53 axis is involved in the growth of human acute myeloid leukemia. Exp Cell Res 365:185–193. https://doi.org/10.1016/j.yexcr.2018.02.036
Chen WD, Zhang JL, Wang XY, Hu ZW, Qian YB (2019) The JAK2/STAT3 signaling pathway is required for inflammation and cell death induced by cerulein in AR42J cells. Eur Rev Med Pharmacol Sci 23:1770–1777. https://doi.org/10.26355/eurrev-201902-17139
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Wu DM, Wang S, Shen M, Wang YJ, Zhang B, Wu ZQ, Lu J, Zheng YL (2017) S100A9 gene silencing inhibits the release of pro-inflammatory cytokines by blocking the IL-17 signalling pathway in mice with acute pancreatitis. J Cell Mol Med 22:2378–2389. https://doi.org/10.1111/jcmm.13532
Sah RP, Dawra RK, Saluja AK (2012) Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol 28:507–515. https://doi.org/10.1097/mog.0b013e3283567f52
Sah RP, Dawra RK, Saluja AK (2013) New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol 29:523–530. https://doi.org/10.1097/mog.0b013e328363e399
Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M (2013) Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 144:1199–1209. https://doi.org/10.1053/j.gastro.2013.02.007
Christophe J (1994) Pancreatic tumoral cell line AR42J: an amphicrine model. Am J Physiol 266:G963–G971. https://doi.org/10.1152/ajpgi.1994.266.6.G963
Landau Z, Forti E, Alcaly M, Birk RZ (2006) Palmitate induced lipoapoptosis of exocrine pancreas AR42J cells. Apoptosis 11:717–724. https://doi.org/10.1007/s10495-006-5425-3
Cai Y, Shen Y, Xu G, Tao R, Yuan W, Huang Z, Zhang D (2016) TRAM1 protects AR42J cells from caerulein-induced acute pancreatitis through ER stress-apoptosis pathway. Vitro Cell Dev Biol Anim 52:530–536. https://doi.org/10.1007/s11626-016-0011-7
Platanitis E, Demiroz D, Schneller A, Fischer K, Capelle C, Hartl M, Gossenreiter T, Müller M, Novatchkova M, Decker T (2019) A molecular switch from STAT2-IRF9 to ISGF3 underlies interferon-induced gene transcription. Nat Commun 10:292. https://doi.org/10.1038/s41467-019-10970-y
Kolosenko FM, Forsberg S, Johnsson P, Cheon H, Holvey-Bates EG, Edsbacker E, Pellegrini P, Rassoolzadeh H, Brnjic S, Larsson R, Stark GR, Grander D, Linder S, Tamm KP, De Milito A (2015) Cell crowding induces interferon regulatory factor 9, which confers resistance to chemotherapeutic drugs. Int J Cancer 15(136):E51–61. https://doi.org/10.1002/ijc.29161
Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T (2003) Integration of interferon-α/β signalling to p53 responses in tumor suppression and antiviral defence. Nature 424:516–523. https://doi.org/10.1038/nature01850
Vonlaufen A, Apte MV, Imhof BA, Frossard JL (2007) The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol 213:239–248. https://doi.org/10.1002/path.2231
Gu H, Werner J, Bergmann F, Whitcomb DC, Büchler MW, Fortunato F (2013) Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis 4:e816. https://doi.org/10.1038/cddis.2013.354
Habtezion A (2015) Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 31:395–399. https://doi.org/10.1097/mog.0000000000000195
Sun YM, Zheng S, Chen X, Gao F, Zhang J (2020) Lower Nr5a2 level downregulates the β-catenin and TCF-4 expression in caerulein-induced pancreatic inflammation. Front Physiol 10:1549. https://doi.org/10.3389/fphys.2019.01549
Shen AH, Kim HJ, Oh GS, Lee SH, Pandit A, Khadka D, Choe SK, Kwak SC, Yang SH, Cho EY, Kim HS, Kim H, Park R, Kwak TH, So HS (2017) NAD+ augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep 7:3006. https://doi.org/10.1038/s41598-017-03418-0
Brooks CL, Gu W (2011) The impact of acetylation and deacetylation on the p53 pathway. Protein Cell 2:456–462. https://doi.org/10.1007/s13238-011-1063-9
Acknowledgements
This work was supported by National Natural Science Foundation Incubation Plan (Grant No. 2019GQFY03) and the Doctoral Research Fund Project of the Second Affiliated Hospital of Anhui Medical University (Grant No. 2014BKJ034).
Author information
Authors and Affiliations
Contributions
B-HX contributed to methodology, software, data curation, and writing-original draft preparation. YL participated in methodology, data curation, and investigation. YS involved in methodology, software, and investigation. HC did investigation. W-LY took part in conceptualization, writing-reviewing and editing, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, BH., Liu, Y., Chen, H. et al. A novel function of IRF9 in acute pancreatitis by modulating cell apoptosis, proliferation, migration, and suppressing SIRT1-p53. Mol Cell Biochem 472, 125–134 (2020). https://doi.org/10.1007/s11010-020-03791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03791-x