Abstract
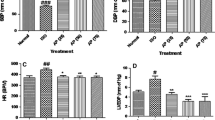
Mitochondrial dysfunction plays crucial role in the pathologenesis of myocardial infarction (MI). The present study evaluated the protective effect of α-bisabolol against isoproterenol (ISO)-induced mitochondrial dysfunction and apoptosis in rats. Male albino Wistar rats were pre- and co-treated with intraperitoneal injection of α-bisabolol (25 mg/kg body weight) daily for 10 days. To induce experimental MI, ISO (85 mg/kg body weight) was injected subcutaneously to the rats at an interval of 24 h for 2 days (9th and 10th day). ISO-induced MI was indicated by the decreased activities of heart creatine kinase and lactate dehydrogenase in rats. ISO administration also enhanced the concentrations of heart mitochondrial lipid peroxidation products and decreased the activities/concentrations of mitochondrial antioxidants, Kreb’s cycle dehydrogenases and mitochondrial electron transport chain complexes I, II + III and IV in rats. Furthermore, ISO triggers calcium overload and ATP depletion in the rat’s heart mitochondria followed by the mitochondrial cytochrome-C release and the activation of intrinsic pathway of apoptosis by upregulating the myocardial pro-apoptotic Bax, P53, APAF-1, active caspase-3, active caspase-9 and down regulating the expressions of anti-apoptotic Bcl-2. α-Bisabolol pre and co-treatment showed considerable protective effects on all the biochemical and molecular parameters studied. Transmission electron microscopic study and mitochondrial swelling assay confirmed our biochemical and molecular findings. The in vitro study on hydroxyl radical also revealed the potent free radical scavenging activity of α-bisabolol. Thus, α-bisabolol attenuates mitochondrial dysfunction and intrinsic pathway of apoptosis in ISO-induced myocardial infarcted rats.








Similar content being viewed by others
Abbreviations
- ISO:
-
Isoproterenol
- MI:
-
Myocardial infarction
- ATP:
-
Adenosine triphosphate
- Bax:
-
B-cell lymphoma-2 associated-x
- APAF-1:
-
Apoptotic protease activating factor 1
- Bcl-2:
-
B-cell lymphoma-2
- ETC:
-
Electron transport chain
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid
- Ca2+ :
-
Calcium
- CK:
-
Creatine kinase
- LDH:
-
Lactate dehydrogenase
- TBARS:
-
Thiobarbituric acid reactive substances
- LOOH:
-
Lipid hydroperoxide
- SOD:
-
Superoxide dismutase
- GSH:
-
Reduced glutathione
- ICDH:
-
Isocitrate dehydrogenase
- MDH:
-
Malate dehydrogenase
- SDH:
-
Succinate dehydrogenase
- α-KGDH:
-
α-Ketoglutarate dehydrogenase
- TEM:
-
Transmission electron microscopy
- OH• :
-
Hydroxyl radical
- DMRT:
-
Duncan’s multiple range test
- SPSS:
-
Statistical Package for the Social Science
- SD:
-
Standard deviation
- NAD:
-
Nicotinamide adenine dinucleotide
- NADH:
-
Reduced nicotinamide adenine dinucleotide
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
References
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2015) Catecholamine toxicity triggers myocardial membrane destabilization in rats: thymol and its counter action. RSC Adv 5:43338–43344. https://doi.org/10.1039/C5RA00903K
Hansi Priscilla D, Stanely Mainzen Prince P (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179:118–124. https://doi.org/10.1016/j.cbi.2008.12.012
Sivakumar R, Anandh Babu PV, Shyamaladevi CS (2008) Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem Biol Interact 176:227–233. https://doi.org/10.1016/j.cbi.2008.08.008
Xiang YK (2011) Compartmentalization of β-adrenergic signals in cardiomyocytes. Circ Res 109:231–244. https://doi.org/10.1161/CIRCRESAHA.110.231340
Xu Q, Dalic A, Fang L et al (2011) Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol 252:1012–1028. https://doi.org/10.1111/j.1476-5381.2010.01043.x
Hemalatha KL, Stanely Mainzen P, Prince (2016) Preventive effects of zingerone on cardiac mitochondrial oxidative stress, calcium ion overload and adenosine triphosphate depletion in isoproterenol induced myocardial infarcted rats. RSC Adv 6:12332. https://doi.org/10.1039/C6RA23330A
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2015) Thymol attenuates altered lipid metabolism in β-adrenergic agonist induced myocardial infarcted rats by inhibiting tachycardia, altered electrocardiogram, apoptosis and cardiac hypertrophy. J Funct Foods 14:51–62. https://doi.org/10.1016/j.jff.2015.01.013
Nagoor Meeran MF, Stanely Mainzen Prince P, Hidhayath Basha R (2012) Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study. Eur J Pharmacol 677:116–122. https://doi.org/10.1016/j.ejphar.2011.11.043
Nagoor Meeran MF, Stanely Mainzen Prince P (2012) Protective effects of N-acetyl cysteine on membrane-bound adenosine triphosphatases and minerals in isoproterenol-induced myocardial-infarcted rats: an in vivo and in vitro study. J Biochem Mol Toxicol 26:276–281. https://doi.org/10.1002/jbt.21419
Rimbaud S, Garnier A, Ventura-Clapier R (2009) Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep 61:131–138. https://doi.org/10.1016/S1734-1140(09)70015-5
Murray AJ, Edwards LM, Clarke K (2007) Mitochondria and heart failure. Curr Opin Clin Nutr Metab Care 10:704–711. https://doi.org/10.1097/MCO.0b013e3282f0ecbe
Mari Kannan M, Darlin Quine S (2012) Ellagic acid protects mitochondria from β-adrenergic agonist induced myocardial damage in rats; evidence from in vivo, in vitro and ultra structural study. Food Res Int 45:1–8. https://doi.org/10.1016/j.foodres.2011.09.018
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Nat Rev Cardiol 9:620–633. https://doi.org/10.1161/CIR.0b013e31826e1058
Hare JM (2001) Oxidative stress and apoptosis in heart failure progression. Circ Res 89:198–200
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
El-Missiry MA, Amer MA, Hemieda FA et al (2015) Cardioameliorative effect of punicalagin against streptozotocin-induced apoptosis, redox imbalance, metabolic changes and inflammation. Egypt J Basic Appl Sci 2:247–260. https://doi.org/10.1016/j.ejbas.2015.09.004
Kamatou GPP, Viljoen AM (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87:1–7. https://doi.org/10.1007/s11746-009-1483-3
Buitrago A, Rojas J, Rojas L et al (2015) Essential oil composition and antimicrobial activity of Vismia macrophylla leaves and fruits collected in Tachira-Venezuela. Nat Prod Commun 10:375–377
Zargaran A, Borhani-Haghighi A, Faridi P et al (2014) Potential effect and mechanism of action of topical chamomile (Matricaria chammomila L.) oil on migraine headache: a medical hypothesis. Med Hypotheses 83:566–569. https://doi.org/10.1016/j.mehy.2014.08.023
Brehm-Stecher BF, Johnson EA (2003) Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol and apritone. Antimicrob. Agents Chemother 47:3357–3360. https://doi.org/10.1128/AAC.47.10.3357-3360.2003
Moura Rocha NF, Venancio ET, Moura BA (2010) Gastroprotection of (-) alpha-bisabolol on acute gastric mucosal lesions in mice: the possible involved pharmacological mechanisms. Fundam Clin Pharmacol 24:63–71
Braga PC, Dal Sasso M, Fonti E et al (2009) Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell free systems. Pharmacology 83:110–115. https://doi.org/10.1159/000186049
Gomes-Carneiro MR, Dias DM, De-Oliveira AC (2005) Evaluation of mutagenic and antimutagenic activities of alpha-bisabolol in the Salmonella⁄microsome assay. Mutat Res 585:105–112
Corpas-Lopez V, Merino-Espinosa G, Lopez-Viota M et al (2016) Topical treatment of Leishmania tropica infection using (-)-α-bisabolol ointment in a Hamster model: effectiveness and safety assessment. J Nat Prod 79:2403–2407. https://doi.org/10.1021/acs.jnatprod.6b00740
Ojha S, Goyal S, Kumari S et al (2012) Pyruvate attenuates cardiac dysfunction and oxidative stress in isoproterenol induced cardiotoxicity. Exp Toxicol Pathol 64:393–399. https://doi.org/10.1016/j.etp.2010.10.004
Ojha S, Bhatia J, Arora S (2011) Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity:a biochemical and histopathological evaluation. J Environ Biol 32:731–738
Ojha S, Goyal S, Sharma C, Arora S, Kumari S, Arya DS (2013) Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats. Human Exp Toxicol 32:492–503
Malik S, Goyal S, Ojha SK et al (2011) Seabuckthorn attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity in rats. Int J Toxicol 30:671–680. https://doi.org/10.1177/1091581811417898
Ojha S, Azimullah S, Al Taee H, Meeran MFN (2017) Cardioprotective effect of (-)-α-Bisabolol in animal model of myocardial infarction. Planta Med Int 4:83. https://doi.org/10.1055/s-0037-1608133
Fraga CG, Leibovitz BE, Toppel AL (1988) Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–251. https://doi.org/10.1016/0891-5849(88)90023-8
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389. https://doi.org/10.1016/0003-2697(92)90122-N
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Sinha K (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
King J (1965) Isocitrate dehydrogenase. In: King JC, Van D (eds) Practical clinical enzymol. Nostrand Co, London, p 363
Mehler H, Kornberg A, Grisolia S et al (1948) The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J Biol Chem 174:961–977
Slater EC, Borner WD Jr (1952) The effect of fluoride on the succinic oxidase system. Biochem J 52:185–196
Reed LJ, Mukherjee RB (1969) α-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Lowenstein JM (ed) Methods in enzymology. Academic Press, London, pp 53–61
Kramer KA, Oglesbee D, Hartman SJ (2005) Automated spectrophotometric analysis of mitochondrial respiratory chain complex enzyme activities in cultured skin fibroblasts. Clin Chem 51:2110–2116. https://doi.org/10.1373/clinchem.2005.050146
Pearl W, Cascarano J, Zweifach BW (1963) Microdetermination of cytochrome oxidase in rat tissues by the oxidation on N-phenyl-p-phenylenediamine or ascorbic acid. J Histochem Cytochem 11:102–104
Williams JR, Coorkey BE (1967) Assay of intermediates of the citric acid cycle and related compounds by flourimetric enzymatic methods. In: Lowenstein JM (ed) Methods in enzymol. Academic Press, New York, pp 488–492
Maloyan A, Sanbe A, Osinska H et al (2005) Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 112:3451–3461. https://doi.org/10.1161/CIRCULATIONAHA.105.572552
Lang RAD (1987). In: Darely-Usmar VM, Reckwood D, Wison MT (eds) Mitochondria, a practical approach. IRL Press Ltd, Washington, pp 17–20
Halliwell B, Gutteridge M, Aruoma OI, The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 255:215–219
Moovendhan M, Seedevi P, Shanmugam A (2016) Exploration of the preventive effect of S. lessoniana liver oil on cardiac markers, hematological patterns and lysosomal hydrolases in isoproterenol-induced myocardial infarction in Wistar rats: a novel report. RSC Adv 6:64147–64154. https://doi.org/10.1039/C6RA11369A
Senthil Kumaran K, Stanely Mainzen Prince P (2010) Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones 15:791–806. https://doi.org/10.1007/s12192-010-0187-9
Zheng XH, Liu CP, Hao ZG et al (2017) Protective effect and mechanistic evaluation of linalool against acute myocardial ischemia and reperfusion injury in rats. RSC Adv 7:34473–34481. https://doi.org/10.1039/C7RA00743D
Basha RH, Priscilla DH (2011) An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Exp Toxicol Pathol 65:7–14. https://doi.org/10.1016/j.etp.2011.05.002
Plaut WE, Cook M, Aogaichi T (1983) The subcellular location of isozymes of NADP-isocitrate dehydrogenase in tissues from pig, ox and rat. Biochim Biophys Acta 760:300–308. https://doi.org/10.1016/0304-4165(83)90177-0
Yogeeta SK, Raghavendran HR, Gnanapragasam A et al (2006) Ferulic acid with ascorbic acid synergistically extenuates the mitochondrial dysfunction during beta-adrenergic catecholamine induced cardio toxicity in rats. Chem Biol Interact 253:250–259. https://doi.org/10.1016/j.cbi.2006.04.018
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2016) Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in β-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chem Biol Interact 244:159–168. https://doi.org/10.1016/j.cbi.2015.12.006
Ambrosio G, Zweier J, Duilio C (1993) Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268:18532–18541
Sudheesh NP, Ajith TA, Janardhanan KK (2013) Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. Int J Cardiol 165:117–125. https://doi.org/10.1016/j.ijcard.2011.07.103
Suchalatha S, Srinivasan P, Shyamala Devi C (2007) Effect of T. chebula on mitochondrial alterations in experimental myocardial injury. Chem Biol Interact 259:145–153. https://doi.org/10.1016/j.cbi.2007.06.034
Ide T, Tsutsui H, Hayashidani S et al (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88:529–535. https://doi.org/10.1161/01.RES.88.5.529
Riba A, Deres L, Sumegi B et al (2017) Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid Med Cell Longev 2017:10. https://doi.org/10.1155/2017/6819281
Starkov AA, Polster BM, Fiskum G (2002) Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem 83:220–228. https://doi.org/10.1046/j.1471-4159.2002.01153.x
Abell ED, Doenst T (2011) Mitochondrial adaptations to physiological vs pathological cardiac hypertrophy. Cardiovasc Res 90:234–242. https://doi.org/10.1093/cvr/cvr015
Wang M, Si JY, Yu Y (2014) Red clover flavonoids protect against oxidative stress-induced cardiotoxicity in vivo and in vitro. RSC Adv 4:54668–54676. https://doi.org/10.1039/C4RA08407A
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2016) Synthetic catecholamine triggers β1-adrenergic receptor activation and stimulates cardiotoxicity via oxidative stress mediated apoptotic cell death in rats: abrogating action of thymol. Chem Biol Interact 251:17–25. https://doi.org/10.1016/j.cbi.2016.03.017
Radhiga T, Rajamanickam C, Sundaresan A (2012) Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie 94:1135–1142. https://doi.org/10.1016/j.biochi.2012.01.015
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619. https://doi.org/10.1016/0092-8674(93)90509-O
Sahu BD, Anubolu H, Koneru M (2014) Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: possible involvement of mitochondrial dysfunction and apoptosis. Life Sci 107:59–67. https://doi.org/10.1016/j.lfs.2014.04.035
Joo JH, Ueda E, Bortner CD et al (2015) Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T-lymphoblastic leukemia Molt4 cells. Biochem Pharmacol 97:256–268. https://doi.org/10.1016/j.bcp.2015.08.086
Zhou B, Wu LJ, Tashiro S et al (2006) Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation of cell cycle. Biol Pharm Bull 29:1900–1905. https://doi.org/10.1248/bpb.29.1900
Wickens P (2011) Ageing and the free radical theory. Respir Physiol 128:379–391. https://doi.org/10.1016/S0034-5687(01)00313-9
Acknowledgements
The authors wish to thank United Arab Emirates University for the funding through University Program for Advanced Research (UPAR) (Grant No. 31M195).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagoor Meeran, M.F., Laham, F., Azimullah, S. et al. α-Bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol Cell Biochem 453, 89–102 (2019). https://doi.org/10.1007/s11010-018-3434-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3434-5