Abstract
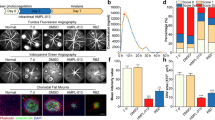
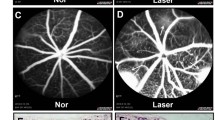
Choroidal neovascularization (CNV) is the hallmark of late-staged wet age-related macular degeneration (AMD). Vascular endothelial growth factor (VEGF) is a key component in the development and progression of wet AMD. DMBT, 6,6′-bis(2,3-dimethoxybenzoyl)-α,α-d-trehalose, had been proved that it could suppress tumor angiogenesis and metastasis by inhibiting production of VEGF. But the effects of DMBT on CNV were not known. This study was to investigate effects and mechanisms of DMBT on CNV in vitro and in vivo. Results showed that DMBT could inhibit migration and tube formation of RF/6A cells under ARPE-19 hypoxia conditioned medium. DMBT could reduce lesion area in laser-induced CNV model mice. ELISA and Western blotting assay showed that DMBT markedly inhibited secretion of VEGF in vitro and in vivo. Furthermore, DMBT restrained ROS level under hypoxia via suppressing Nrf2/HO-1 pathway. DMBT effectively suppressed hypoxia-induced the up-regulation of p-Akt, p-NF-κB, and HIF-1α. These results suggest that DMBT can inhibit CNV by down-regulation of VEGF in retina through Akt/NF-κB/HIF-1α and ERK/Nrf2/HO-1/HIF-1α pathway. DMBT might be a promising lead molecule for anti-CNV and serve as a therapeutic agent to inhibit CNV.









Similar content being viewed by others
References
Paeng SH, Jung WK, Park WS, Lee DS, Kim GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, Park S, Choi IW (2015) Caffeic acid phenethyl ester reduces the secretion of vascular endothelial growth factor through the inhibition of the ROS, PI3K and HIF-1α signaling pathways in human retinal pigment epithelial cells under hypoxic conditions. Int J Mol Med 35:1419–1426
Pennington KL, DeAngelis MM (2016) Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis 3:34
Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K (2009) Regulatory role of HIF-1α in the pathogenesis of age-related macular degeneration (AMD). Ageing Res Rev 8:349–358
Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K (2012) Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol 90:299–309
Wang H, Hartnett ME (2016) Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis 22:189–202
Bhutto I, Lutty G (2012) Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med 33:295–317
de Jong PT (2006) Age-related macular degeneration. N Engl J Med 355:1474–1485
Nowak JZ (2014) AMD–the retinal disease with an unprecised etiopathogenesis: in search of effective therapeutics. Acta Pol Pharm 71:900–916
Campa C, Harding SP (2011) Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets 12:173–181
Al-Latayfeh M, Silva PS, Sun JK, Aiello LP (2012) Antiangiogenic therapy for ischemic retinopathies. Cold Spring Harb Perspect Med 2:a006411
Rosen R, Vagaggini T, Chen Y, Hu DN (2015) Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells through decreased protein levels of hypoxia-inducible factors-1α. Biomed Res Int 2015:687386
Kurihara T, Westenskow PD, Friedlander M (2014) Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv Exp Med Biol 801:275–281
Fanjul-moles ML, López-riquelme GO (2016) Relationship between oxidative stress, circadian rhythms, and AMD. Oxid Med Cell Longev 2016:7420637
Handa JT (2012) How does the macula protect itself from oxidative stress? Mol Aspects Med 33:418–435
Igarashi Y, Mogi T, Yanase S, Miyanaga S, Fujita T, Sakurai H, Saiki I, Ohsaki A (2009) Brartemicin, an inhibitor of tumor cell invasion from the actinomycete Nonomuraea sp. J Nat Prod 72:980–982
Tang L, Yue B, Cheng Y, Yao H, Ma X, Tian Q, Ge L, Liu Z, Han X (2014) Inhibition of invasion and metastasis by DMBT, a novel trehalose derivative, through Akt/GSK-3β/β-catenin pathway in B16BL6 cells. Chem Biol Interact 222:7–17
Tang L, Ma X, Tian Q, Cheng Y, Yao H, Liu Z, Qu X, Han X (2013) Inhibition of angiogenesis and invasion by DMBT is mediated by downregulation of VEGF and MMP-9 through Akt pathway in MDA-MB-231 breast cancer cells. Food Chem Toxicol 56:204–213
Jiang YL, Tang LQ, Miyanaga S, Igarashi Y, Saiki I, Liu ZP (2011) Synthesis and evaluation of trehalose-based compounds as anti-invasive agents. Bioorg Med Chem Lett 21:1089–1091
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379:1728–1738
Kim JH, Choi YK, Lee KS, Cho DH, Baek YY, Lee DK, Ha KS, Choe J, Won MH, Jeoung D, Lee H, Kwon YG, Kim YM (2012) Functional dissection of Nrf2-dependent phase II genes in vascular inflammation and endotoxic injury using Keap1 siRNA. Free Radic Biol Med 53:629–640
Campochiaro PA (2013) Ocular neovascularization. J Mol Med 91:311–321
Kim JH, Lee KS, Lee DK, Kim J, Kwak SN, Ha KS, Choe J, Won MH, Cho BR, Jeoung D, Lee H, Kwon YG, Kim YM (2014) Hypoxia-responsive microRNA-101 promotes angiogenesis via heme oxygenase-1/vascular endothelial growth factor axis by targeting cullin 3. Antioxid Redox Signal 21:2469–2482
Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, Shyy JY (2013) Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest 123:1057–1067
Wu H, Zhao J, Chen M, Wang H, Yao Q, Fan J, Zhang M (2017) The anti-aging effect of erythropoietin via the ERK/Nrf2-ARE pathway in aging rats. J Mol Neurosci 61:449–458
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73:3221–3247
Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K (2008) Interaction of aging-associated signaling cascades: inhibition of NF-kappa B signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 65:1049–1058
Luyckx J, Baudouin C (2011) Trehalose: an intriguing disaccharide with potential for medical application in ophthalmology. Clin Ophthalmol 5:577–581
Mateo Orobia AJ, Casas Pascual P, Cristóbal Bescós J, Perez García D, Peiro Embid C, Del Buey Sayas M, Korobko Kulikova V, Ojeda NL (2017) Effects of 3% trehalose as an adjuvant treatment after LASIK. Clin Ophthalmol 11:347–353
Takeuchi K, Nakazawa M, Ebina Y (2011) Effects of trehalose on VEGF-stimulated angiogenesis and myofibroblast proliferation: implications for glaucoma filtration surgery. Invest Ophthalmol Vis Sci 52:6987–6993
Takeuchi K, Nakazawa M, Ebina Y, Sato K, Metoki T, Miyagawa Y, Ito T (2010) Inhibitory effects of trehalose on fibroblast proliferation and implications for ocular surgery. Exp Eye Res 91:567–577
Wu J, Ke X, Wang W, Zhang H, Ma N, Fu W, Zhao M, Gao X, Hao X, Zhang Z (2016) Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1α/VEGF pathway. Int J Biol Sci 12:1363–1371
Kim SH, Kim H, Ku HJ, Park JH, Cha H, Lee S, Lee JH, Park JW (2016) Oxalomalate reduces expression and secretion of vascular endothelial growth factor in the retinal pigment epithelium and inhibits angiogenesis: implications for age-related macular degeneration. Redox Biol 10:211–220
Wang H, Han X, Wittchen ES, Hartnett ME (2016) TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol Vis 22:116–128
Park H, Lee DS, Yim MJ, Choi YH, Park S, Seo SK, Choi JS, Jang WH, Yea SS, Park WS, Lee CM, Jung WK, Choi IW (2015) 3,3′-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions. Int J Mol Med 36:301–308
Russo MA, Sansone L, Carnevale I, Limana F, Runci A, Polletta L, Perrone GA, De Santis E, Tafani M (2015) One special question to start with: can HIF/NF-κB be a target in inflammation? Endocr Metab Immune Disord Drug Targets 15:171–185
Cervellati F, Cervellati C, Romani A, Cremonini E, Sticozzi C, Belmonte G, Pessina F, Valacchi G (2014) Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic Res 48:303–312
Donato AJ, Morgan RG, Walker AE, Lesniewski LA (2015) Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol 89:122–135
Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (No. ZR2013HM084) and Key Research and Development Program of Shandong Province (2016GSF201152) of P. R. China. Compounds DMBT used in this study were synthesized by Prof. Zhaopeng Liu, Department of Medicinal Chemistry, School of Pharmaceutical Sciences, Shandong University. Thanks are due to Dr. Yanai at the Department of Ophthalmology, Graduate School of Medicine, Yamaguchi University, for his help and guide in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, S., Zhou, Y., Zhou, L. et al. Anti-neovascularization effects of DMBT in age-related macular degeneration by inhibition of VEGF secretion through ROS-dependent signaling pathway. Mol Cell Biochem 448, 225–235 (2018). https://doi.org/10.1007/s11010-018-3328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3328-6