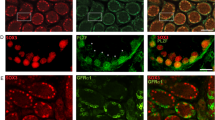
Abstract
SRY-related box (Sox) transcription factors are conserved among vertebrate species. These proteins regulate multiple processes including sex determination and testis differentiation of the male embryo. Members of the Sox family have been identified in pre- and postnatal testis and are known to play an important role in sex determination (Sry, Sox9), male gonadal development, and fertility (Sox4, Sox8, Sox30). However, their expression profiles per cell types remain elusive. The objectives of this research were to characterize the expression profiles of Sox family members within adult testes using publically available datasets and to determine whether these findings are consistent with literature as well as immunofluorescence and in situ hybridization results. We have found that Sox4, Sox8, Sox9, and Sox12 are highly expressed in Sertoli cells, whereas Sox5, Sox6, and Sox30 were typically expressed in spermatocytes and spermatids. Spermatogonia were characterized by the expressions of Sox3, Sox4, Sox12, Sox13, and Sox18. Hence, these results suggest that Sox transcription factors may play different roles according to cell types of the adult mammalian testis.




Similar content being viewed by others
References
Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 3:167–170
Grosschedl R, Giese K, Pagel J (1994) HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet TIG 10:94–100
Bowles J, Schepers G, Koopman P (2000) Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol 227:239–255. https://doi.org/10.1006/dbio.2000.9883
Gubbay J, Collignon J, Koopman P et al (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346:245–250. https://doi.org/10.1038/346245a0
Koopman P, Gubbay J, Vivian N et al (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121. https://doi.org/10.1038/351117a0
Sinclair AH, Berta P, Palmer MS et al (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244. https://doi.org/10.1038/346240a0
Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934. https://doi.org/10.1038/nature06944
Barrionuevo F, Bagheri-Fam S, Klattig J et al (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74:195–201. https://doi.org/10.1095/biolreprod.105.045930
Chaboissier M-C, Kobayashi A, Vidal VIP et al (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Dev Camb Engl 131:1891–1901. https://doi.org/10.1242/dev.01087
DeFalco T, Takahashi S, Capel B (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14–26. https://doi.org/10.1016/j.ydbio.2011.01.011
Yao HH-C, Whoriskey W, Capel B (2002) Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440. https://doi.org/10.1101/gad.981202
Barrios F, Filipponi D, Pellegrini M et al (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123:871–880. https://doi.org/10.1242/jcs.057968
Bowles J, Feng C-W, Spiller C et al (2010) FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 19:440–449. https://doi.org/10.1016/j.devcel.2010.08.010
Foster JW, Dominguez-Steglich MA, Guioli S et al (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525–530. https://doi.org/10.1038/372525a0
Wagner T, Wirth J, Meyer J et al (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120
Wright E, Hargrave MR, Christiansen J et al (1995) The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet 9:15–20. https://doi.org/10.1038/ng0195-15
Bi W, Deng JM, Zhang Z et al (1999) Sox9 is required for cartilage formation. Nat Genet 22:85–89. https://doi.org/10.1038/8792
Bi W, Huang W, Whitworth DJ et al (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA 98:6698–6703. https://doi.org/10.1073/pnas.111092198
Schafer AJ, Foster JW, Kwok C et al (1996) Campomelic dysplasia with XY sex reversal: diverse phenotypes resulting from mutations in a single gene. Ann N Y Acad Sci 785:137–149
Barrionuevo F, Georg I, Scherthan H et al (2009) Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 327:301–312. https://doi.org/10.1016/j.ydbio.2008.12.011
O’Bryan MK, Takada S, Kennedy CL et al (2008) Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol 316:359–370. https://doi.org/10.1016/j.ydbio.2008.01.042
Dy P, Penzo-Méndez A, Wang H et al (2008) The three SoxC proteins—Sox4, Sox11 and Sox12—exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res 36:3101–3117. https://doi.org/10.1093/nar/gkn162
Zhao L, Arsenault M, Ng ET et al (2017) SOX4 regulates gonad morphogenesis and promotes male germ cell differentiation in mice. Dev Biol 423:46–56. https://doi.org/10.1016/j.ydbio.2017.01.013
van de Wetering M, Oosterwegel M, van Norren K, Clevers H (1993) Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J 12:3847–3854
Denny P, Swift S, Connor F, Ashworth A (1992) An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J 11:3705–3712
Brawand D, Soumillon M, Necsulea A et al (2011) The evolution of gene expression levels in mammalian organs. Nature 478:343–348. https://doi.org/10.1038/nature10532
Connor F, Wright E, Denny P et al (1995) The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res 23:3365–3372
Daigle M, Roumaud P, Martin LJ (2015) Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Mol Cell Biochem 407:209–221. https://doi.org/10.1007/s11010-015-2470-7
Osaki E, Nishina Y, Inazawa J et al (1999) Identification of a novel Sry-related gene and its germ cell-specific expression. Nucleic Acids Res 27:2503–2510
Han F, Dong Y, Liu W et al (2014) Epigenetic regulation of Sox30 is associated with testis development in mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0097203
Uhlén M, Fagerberg L, Hallström BM et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347:1260419. https://doi.org/10.1126/science.1260419
Petit FG, Kervarrec C, Jamin SP et al (2015) Combining RNA and protein profiling data with network interactions identifies genes associated with spermatogenesis in mouse and human. Biol Reprod 92:71. https://doi.org/10.1095/biolreprod.114.126250
Raverot G, Weiss J, Park SY et al (2005) Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev Biol 283:215–225. https://doi.org/10.1016/j.ydbio.2005.04.013
Kanai Y, Kanai-Azuma M, Noce T et al (1996) Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol 133:667–681
Namekawa SH, Park PJ, Zhang L-F et al (2006) Postmeiotic sex chromatin in the male germline of mice. Curr Biol CB 16:660–667. https://doi.org/10.1016/j.cub.2006.01.066
Tsai-Morris C-H, Sato H, Gutti R, Dufau ML (2012) Role of gonadotropin regulated testicular RNA helicase (GRTH/Ddx25) on polysomal associated mRNAs in mouse testis. PloS ONE 7:e32470. https://doi.org/10.1371/journal.pone.0032470
Soumillon M, Necsulea A, Weier M et al (2013) Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep 3:2179–2190. https://doi.org/10.1016/j.celrep.2013.05.031
Chocu S, Evrard B, Lavigne R et al (2014) Forty-four novel protein-coding loci discovered using a proteomics informed by transcriptomics (PIT) approach in rat male germ cells. Biol Reprod 91:123. https://doi.org/10.1095/biolreprod.114.122416
Chalmel F, Lardenois A, Evrard B et al (2014) High-resolution profiling of novel transcribed regions during rat spermatogenesis. Biol Reprod 91:5. https://doi.org/10.1095/biolreprod.114.118166
Kim D, Pertea G, Trapnell C et al (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. https://doi.org/10.1186/gb-2013-14-4-r36
Trapnell C, Williams BA, Pertea G et al (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. https://doi.org/10.1038/nbt.1621
Yuan L, Liu JG, Zhao J et al (2000) The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 5:73–83
Chang H, Gao F, Guillou F et al (2008) Wt1 negatively regulates beta-catenin signaling during testis development. Dev Camb Engl 135:1875–1885. https://doi.org/10.1242/dev.018572
Matheu A, Maraver A, Collado M et al (2009) Anti-aging activity of the Ink4/Arf locus. Aging Cell 8:152–161. https://doi.org/10.1111/j.1474-9726.2009.00458.x
Wan Y-P, Xi M, He H-C et al (2017) Expression and clinical significance of SOX9 in renal cell carcinoma, bladder cancer and penile cancer. Oncol Res Treat 40:15–20. https://doi.org/10.1159/000455145
Ma F, Ye H, He HH et al (2016) SOX9 drives WNT pathway activation in prostate cancer. J Clin Invest 126:1745–1758. https://doi.org/10.1172/JCI78815
Schilham MW, Oosterwegel MA, Moerer P et al (1996) Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 380:711–714. https://doi.org/10.1038/380711a0
Sock E, Rettig SD, Enderich J et al (2004) Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol 24:6635–6644. https://doi.org/10.1128/MCB.24.15.6635-6644.2004
Hoser M, Potzner MR, Koch JMC et al (2008) Sox12 deletion in the mouse reveals nonreciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol 28:4675–4687. https://doi.org/10.1128/MCB.00338-08
Collignon J, Sockanathan S, Hacker A et al (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122:509–520
Shen JH-C, Ingraham HA (2002) Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol Endocrinol 16:529–540. https://doi.org/10.1210/mend.16.3.0782
Weiss J, Meeks JJ, Hurley L et al (2003) Sox3 Is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23:8084–8091. https://doi.org/10.1128/MCB.23.22.8084-8091.2003
Laronda MM, Jameson JL (2011) Sox3 functions in a cell-autonomous manner to regulate spermatogonial differentiation in mice. Endocrinology 152:1606–1615. https://doi.org/10.1210/en.2010-1249
Wu X, Schmidt JA, Avarbock MR et al (2009) Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci USA 106:21672–21677. https://doi.org/10.1073/pnas.0912432106
Bastian F, Parmentier G, Roux J et al (2008) Bgee: integrating and comparing heterogeneous transcriptome data among species. In: Bairoch A, Cohen-Boulakia S, Froidevaux C (eds) Data integration in the life sciences, Springer, Berlin, pp 124–131
Azuma T, Seki N, Yoshikawa T et al (2000) cDNA cloning, tissue expression, and chromosome mapping of human homolog of SOX18. J Hum Genet 45:192–195. https://doi.org/10.1007/s100380050210
Connor F, Cary PD, Read CM et al (1994) DNA binding and bending properties of the post-meiotically expressed Sry-related protein Sox-5. Nucleic Acids Res 22:3339–3346
Blaise R, Grober J, Rouet P et al (1999) Testis expression of hormone-sensitive lipase is conferred by a specific promoter that contains four regions binding testicular nuclear proteins. J Biol Chem 274:9327–9334
Budde LM, Wu C, Tilman C et al (2002) Regulation of IkappaBbeta expression in testis. Mol Biol Cell 13:4179–4194. https://doi.org/10.1091/mbc.01-07-0373
Moretti C, Mencacci C, Frajese GV et al (2002) Growth hormone-releasing hormone and pituitary adenylate cyclase-activating polypeptide in the reproductive system. Trends Endocrinol Metab TEM 13:428–435
Kiselak EA, Shen X, Song J et al (2010) Transcriptional regulation of an axonemal central apparatus gene, sperm-associated antigen 6, by a SRY-related high mobility group transcription factor, S-SOX5. J Biol Chem 285:30496–30505. https://doi.org/10.1074/jbc.M110.121590
Xu W, Zhang S, Qiu W et al (2009) Spermatogenesis-related ring finger gene ZNF230 promoter: identification and functional analysis. Mol Biol Rep 36:1187–1193. https://doi.org/10.1007/s11033-008-9296-2
Herlofsen SR, Küchler AM, Melvik JE, Brinchmann JE (2011) Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng A 17:1003–1013. https://doi.org/10.1089/ten.tea.2010.0499
Takamatsu N, Kanda H, Tsuchiya I et al (1995) A gene that is related to SRY and is expressed in the testes encodes a leucine zipper-containing protein. Mol Cell Biol 15:3759–3766
Narahara M, Yamada A, Hamada-Kanazawa M et al (2002) cDNA cloning of the Sry-related gene Sox6 from rat with tissue-specific expression. Biol Pharm Bull 25:705–709. https://doi.org/10.1248/bpb.25.705
Hamada-Kanazawa M, Ishikawa K, Ogawa D et al (2004) Suppression of Sox6 in P19 cells leads to failure of neuronal differentiation by retinoic acid and induces retinoic acid-dependent apoptosis. FEBS Lett 577:60–66. https://doi.org/10.1016/j.febslet.2004.09.063
Hamada-Kanazawa M, Ogawa D, Takano M, Miyake M (2016) Sox6 suppression induces RA-dependent apoptosis mediated by BMP-4 expression during neuronal differentiation in P19 cells. Mol Cell Biochem 412:49–57. https://doi.org/10.1007/s11010-015-2607-8
Busada JT, Geyer CB (2016) The role of retinoic acid (RA) in spermatogonial differentiation. Biol Reprod 94:. https://doi.org/10.1095/biolreprod.115.135145
Wang S, Wang X, Ma L et al (2016) Retinoic acid is sufficient for the in vitro induction of mouse spermatocytes. Stem Cell Rep 7:80–94. https://doi.org/10.1016/j.stemcr.2016.05.013
Piskacek S, Gregor M, Nemethova M et al (2007) Nine-amino-acid transactivation domain: establishment and prediction utilities. Genomics 89:756–768. https://doi.org/10.1016/j.ygeno.2007.02.003
Piskacek M, Havelka M, Rezacova M, Knight A (2016) The 9aaTAD transactivation domains: from Gal4 to p53. PLoS ONE. https://doi.org/10.1371/journal.pone.0162842
Rossi P, Dolci S, Albanesi C et al (1993) Direct evidence that the mouse sex-determining gene Sry is expressed in the somatic cells of male fetal gonads and in the germ cell line in the adult testis. Mol Reprod Dev 34:369–373. https://doi.org/10.1002/mrd.1080340404
Salas-Cortés L, Jaubert F, Barbaux S et al (1999) The human SRY protein is present in fetal and adult Sertoli cells and germ cells. Int J Dev Biol 43:135–140
Modi D, Shah C, Sachdeva G et al (2005) Ontogeny and cellular localization of SRY transcripts in the human testes and its detection in spermatozoa. Reprod Camb Engl 130:603–613. https://doi.org/10.1530/rep.1.00413
Zwingman T, Fujimoto H, Lai LW et al (1994) Transcription of circular and noncircular forms of Sry in mouse testes. Mol Reprod Dev 37:370–381. https://doi.org/10.1002/mrd.1080370403
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada (Grant No.: 386557-2012 to L.J.M.) and the New Brunswick Innovation Foundation (NBIF) (Grant No.: IAR2013-029 to L.J.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that would prejudice there impartiality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2018_3302_MOESM1_ESM.pptx
Fig. S1—Negative controls for immunofluorescence detections. Immunofluorescence was performed on adult mouse testis (P120) sections using a rabbit normal IgG (green fluorescence) and a mouse normal IgG (red fluorescence). Cell nuclei were detected using Hoechst 33342 (blue fluorescence). Results are representative of three independent experiments. Magnification, x 200 and x 400 (PPTX 1324 KB)
Rights and permissions
About this article
Cite this article
Roumaud, P., Haché, J. & Martin, L.J. Expression profiles of Sox transcription factors within the postnatal rodent testes. Mol Cell Biochem 447, 175–187 (2018). https://doi.org/10.1007/s11010-018-3302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3302-3