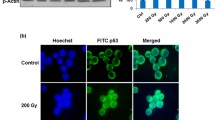
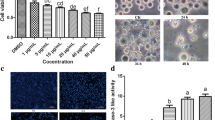
Abstract
Insect cell lines have been utilized as an important higher eukaryotic model system to decipher stress responses and cell death mechanisms. Lepidopteran Sf9 cells (derived from the ovaries of Spodoptera frugiperda) display nearly 100 times higher resistance to ionizing radiation in contrast to mammalian cells, which is partly contributed by an unusually high HDAC activity. However, their response to HDAC inhibition remains to be evaluated. In the present study, the effects of HDAC inhibitor (NaBt) on Sf9 cellular/nuclear morphology, cell cycle progression, DNA damage/repair, redox status, and mitochondrial perturbations were evaluated. NaBt-induced apoptosis was evident at 18 h in Sf9 cells at 2 mM concentration, primarily through mitochondrial induction of oxidative stress and subsequent DNA damage. Cell cycle analysis revealed appearance of sub-G1 DNA content at 12 h onwards and DNA fragmentation by 18 h. Initial few hours of treatment caused significant loss in MMP through oxidation of mitochondrial inner membrane protein, i.e., cardiolipin. HDAC inhibition-mediated apoptosis was associated with increased Bax/Bcl2 ratio, mitochondrial cytochrome-c release, and caspase-3 activation. The study thus infers that Sf9 cells, which can withstand very high radiation doses, are quite sensitive to the increase in the chromatin acetylation levels. In addition, HDAC inhibition also sensitized Sf9 cells to radiation-induced DNA damage, further corroborating our recent finding that chromatin compactness contributes significantly to their radioresistance. Therefore, the study demonstrates prominence of prevailing DNA/chromatin protective mechanisms in Lepidopteran insect cells.






Similar content being viewed by others
Abbreviations
- Sf9:
-
Spodoptera frugiperda-9
- ROS:
-
Reactive oxygen species
- MMP:
-
Mitochondrial membrane potential
- NaBt:
-
Sodium butyrate
- HDACs:
-
Histone deacetylases
References
Koval TM (1991) Gamma-ray and UV sensitive strains of a radioresistant cell line: isolation and cross-sensitivity to other agents. Radiat Res 127:58–63
Koval TM (1991) Recovery from exposure to DNA-damaging chemicals in radiation-resistant insect cells. Mutat Res 262:219–225
Kumar JS, Suman S, Singh V, Chandna S (2012) Radioresistant Sf9 insect cells display moderate resistance against cumene hydroperoxide. Mol Cell Biochem 367:141–151
Clem RJ, Miller LK (1994) Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol 14:5212–5222
Kumarswamy R, Seth RK, Dwarakanath BS, Chandna S (2009) Mitochondrial regulation of insect cell apoptosis: evidence for permeability transition pore-independent cytochrome-c release in the Lepidopteran Sf9 cells. Int J Biochem Cell Biol 41:1430–1440
Kumarswamy R, Chandna S (2010) Inhibition of microRNA-14 contributes to actinomycin-D-induced apoptosis in the Sf9 insect cell line. Cell Biol Int 34:851–857
Chandna S (2010) RE: Multiple factors conferring high radioresistance in insect Sf9 cells. Mutagenesis 25:431–432
Kumar A, Ghosh S, Chandna S (2015) Evidence for microRNA-31 dependent Bim-Bax interaction preceding mitochondrial Bax translocation during radiation-induced apoptosis. Sci Rep 5:15923
Chandna S, Dwarakanath BS, Seth RK, Khaitan D, Adhikari JS, Jain V (2004) Radiation responses of Sf9, a highly radioresistant lepidopteran insect cell line. Int J Radiat Biol 80:301–315
Ljungman M, Nyberg S, Nygren J, Eriksson M, Ahnström G (1991) DNA-bound proteins contribute much more than soluble intracellular compounds to the intrinsic protection against radiation-induced DNA strand breaks in human cells. Radiat Res 127:171–176
Ljungman M (1991) The influence of chromatin structure on the frequency of radiation-induced DNA strand breaks: a study using nuclear and nucleoid monolayers. Radiat Res 126:58–64
Ljungman M, Hanawalt PC (1992) Efficient protection against oxidative DNA damage in chromatin. Mol Carcinog 5:264–269
Kornberg RD, Lorch Y (1992) Chromatin structure and transcription. Annu Rev Cell Biol 8:563–587
Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128:707–719
Adam S, Dabin J, Polo SE (2015) Chromatin plasticity in response to DNA damage: the shape of things to come. DNA Repair 32:120–126
Xu Y, Price BD (2011) Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle 10:261–267
Tsukiyama T (2002) The in vivo functions of ATP-dependent chromatin-remodelling factors. Nat Rev Mol Cell Biol 3:422–429
Vignali M, Hassan AH, Neely KE, Workman JL (2000) ATP-dependent chromatin-remodeling complexes. Mol Cell Biol 20:1899–1910
Sharma K, Kumar A, Chandna S (2016) Constitutive hyperactivity of histone deacetylases enhances radioresistance in Lepidopteran Sf9 insect cells. Biochim Biophys Acta 1860:1237–1246
Suman S, Seth RK, Chandna S (2011) A calcium-insensitive attenuated nitrosative stress response contributes significantly in the radioresistance of Sf9 insect cells. Int J Biochem Cell Biol 43:1340–1353
Suman S, Pandey A, Chandna S (2012) An improved non-enzymatic “DNA ladder assay” for more sensitive and early detection of apoptosis. Cytotechnology 64:9–14
Chandna S (2004) Single-cell gel electrophoresis assay monitors precise kinetics of DNA fragmentation induced during programmed cell death. Cytometry A 61:127–133
López-Larraza DM, Padrón J, Ronci NE, Vidal Rioja LA (2006) Chromatin condensation and differential sensitivity of mammalian and insect cells to DNA strand breaks induced by bleomycin. Mutat Res 600:93–101
Frank CL, Manandhar D, Gordân R, Crawford GE (2016) HDAC inhibitors cause site-specific chromatin remodeling at PU.1-bound enhancers in K562 cells. Epigenetics Chromatin 9:15
Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr 133:2485S-2493S
Rosato RR, Grant S (2005) Histone deacetylase inhibitors: insights into mechanisms of lethality. Expert Opin Ther Targets 9:809–824
Chen Q, Chai YC, Mazumder S et al (2003) The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10:323–334
Ogura A, Oowada S, Kon Y et al (2009) Redox regulation in radiation-induced cytochrome c release from mitochondria of human lung carcinoma A549 cells. Cancer Lett 277:64–71
Hambarde S, Singh V, Chandna S (2013) Evidence for involvement of cytosolic thioredoxin peroxidase in the excessive resistance of Sf9 Lepidopteran insect cells against radiation-induced apoptosis. PLoS ONE 8:e58261
Suman S, Seth RK, Chandna S (2009) Mitochondrial antioxidant defence in radio-resistant Lepidopteran insect cells. Bioinformation 4:19–23
Suman S, Khan Z, Zarin M, Chandna S, Seth RK (2015) Radioresistant Sf9 insect cells display efficient antioxidant defence against high dose γ-radiation. Int J Radiat Biol 91:732–741
Rosato RR, Almenara JA, Dai Y, Grant S (2003) Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther 2:1273–1284
Rosato RR, Almenara JA, Grant S (2003) The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res 63:3637–3645
Falkenberg KJ, Johnstone RW (2014) Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 13:673–691
Robert C, Rassool FV (2012) HDAC inhibitors: roles of DNA damage and repair. Adv Cancer Res 116:87–129
Kumarswamy R, Chandna S (2009) Putative partners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or none of them. Mitochondrion 9:1–8
Kroemer G, Petit P, Zamzami N, Vayssière JL, Mignotte B (1995) The biochemistry of programmed cell death. FASEB J 9:1277–1287
Koval TM, Kazmar ER (1988) DNA double-strand break repair in eukaryotic cell lines having radically different radiosensitivities. Radiat Res 113(2):268–277
Cheng IC, Lee HJ, Wang TC (2009) Multiple factors conferring high radioresistance in insect Sf9 cells. Mutagenesis 24(3):259–269
Suman S, Khaitan D, Pati U, Seth RK, Chandna S (2009) Stress response of a p53 homologue in the radioresistant Sf9 insect cells. Int J Radiat Biol 85(3):238–249
Cerna D, Camphausen K, Tofilon PJ (2006) Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol 73:173–204
Neuzil J, Swettenham E, Gellert N (2004) Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-xL down-regulation. Biochem Biophys Res Commun 314:186–191
Karagiannis TC, Harikrishnan KN, El-Osta A (2005) The histone deacetylase inhibitor, Trichostatin A, enhances radiation sensitivity and accumulation of gammaH2A.X. Cancer Biol Ther 4:787–793
Downs JA, Nussenzweig MC, Nussenzweig A (2007) Chromatin dynamics and the preservation of genetic information. Nature 447:951–958
Acknowledgements
This study was funded through DRDO (Defence Research and Development Organization) Grant# INM-311.1.5. JSK received research fellowship/associateship from Indian Council of Medical Research (ICMR)/Defence Research and Development Organization (DRDO), India during the course of this study.
Author information
Authors and Affiliations
Contributions
SC and JSK designed the study; JSK conducted the experiments; SC, SS, and JSK analyzed data; SC provided materials and supplies; JSK, SS, and SC wrote and approved this manuscript for final submission.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Kumar, J.S., Suman, S. & Chandna, S. Radioresistant Sf9 insect cells readily undergo an intrinsic mode of apoptosis in response to histone deacetylase (HDAC) inhibition. Mol Cell Biochem 444, 207–218 (2018). https://doi.org/10.1007/s11010-017-3245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3245-0