Abstract
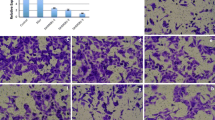
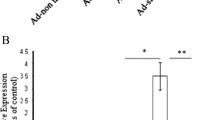
Vascular smooth muscle cells (VSMCs) proliferation is a key process in atherosclerosis. However, little is known about the underlying mechanisms, leading to a lack of effective therapy. This study was to investigate whether dopamine receptor 1 (DR1) is involved in the VSMCs proliferation and related mechanisms. A7r5 cells were treated with oxidized low-density lipoprotein (ox-LDL, 10, 20, 50, 100, 200 µg/mL) in the presence or absence of the SKF38393 (DR1agonist), SCH23390 (DR1antiagonist), SP600125 (JNK inhibitor), PD98059(ERK1/2 inhibitor) or NAC (ROS inhibitor). Cell proliferation and related signaling pathway were evaluated. The expression of DR1 was negatively correlated with increasing of cell proliferation caused by ox-LDL. Cell proliferation and ROS generation in response to ox-LDL were prevented by DR1 agonist or over-expression. The peroxiredoxins protein (Prx1, 2, 3, 5, 6) were increased in A7r5 cells treated with ox-LDL; however, only Prx3 dramatically increased after activation of DR1 compared with ox-LDL group, which is related to activation of JNK/c-Jun pathway. In addition, ERK is associated with the restraining effects of DR1 activation. DR1 activation inhibits VSMCs proliferation primarily by JNK/c-Jun dependent increasing of Prx3, suggesting DR1 a potential target for the prevention of vascular proliferation disease.






Similar content being viewed by others
References
Cai Y, Nagel DJ, Zhou Q, Cygnar KD, Zhao H, Li F, Pi X, Knight PA, Yan C (2015) Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ Res 116(7):1120–1132
Rudijanto A (2007) The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones 39(2):86–93
Baumer Y, McCurdy S, Alcala M, Mehta N, Lee BH, Ginsberg MH, Boisvert WA (2017) CD98 regulates vascular smooth muscle cell proliferation in atherosclerosis. Atherosclerosis 256:105–114
Zhang F, Ren X, Zhao M, Zhou B, Han Y (2016) Angiotensin(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep 30(6):34621
Chen Z, Liu S, Cai Y, Xie K, Zhang W, Dong L, Liu Y, Zheng F, Dun Y, Li N (2016) Suppressive effect of formononetin on platelet-derived growth factor-BB-stimulated proliferation and migration of vascular smooth muscle cells. Exp Ther Med 12(3):1901–1907
Yang J, Yu J, Li D, Yu S, Ke J, Wang L, Wang Y, Qiu Y, Gao X, Zhang J, Huang L (2017) Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL exposure. Autophagy 13(1):82–98
Hu Y, Liu K, Yan M, Zhang Y, Wang Y, Ren L (2016) Icariin inhibits oxidized low-density lipoprotein-induced proliferation of vascular smooth muscle cells by suppressing activation of extracellular signal-regulated kinase 1/2 and expression of proliferating cell nuclear antigen. Mol Med Rep 13(3):2899–2903
Zhang L, Zhang H, Li X, Jia B, Yang Y, Zhou P, Li P, Chen J (2016) Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway. Phytomedicine 23(14):1806–1813
Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL (2007) Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis 190(1):124–134
Di Pietro N, Formoso G, Pandolfi A (2016) Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol 84:1–7
Singh R, Devi S, Gollen R (2015) Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev 31(2):113–126
Goncharov NV, Avdonin PV, Nadeev AD, Zharkikh IL, Jenkins RO (2015) Reactive oxygen species in pathogenesis of atherosclerosis. Curr Pharm Des 21(9):1134–1146
Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, Doroshow JH (2015) NADPH oxidases and cancer. Clin Sci 128(12):863–875
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26
Kim SU, Park YH, Kim JM, Sun HN, Song IS, Huang SM, Lee SH, Chae JI, Hong S, Sik Choi S, Choi SC, Lee TH, Kang SW, Rhee SG, Chang KT, Lee SH, Yu DY, Lee DS (2014) Dominant role of peroxiredoxin/JNK axis in stemness regulation during neurogenesis from embryonic stem cells. Stem Cells 32(4):998–1011
Lee KP, Shin YJ, Cho SC, Lee SM, Bahn YJ, Kim JY, Kwon ES, Jeong DY, Park SC, Rhee SG, Woo HA, Kwon KS (2014) Peroxiredoxin 3 has a crucial role in the contractile function of skeletal muscle by regulating mitochondrial homeostasis. Free Radic Biol Med 77:298–306
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24(1):125–132
Kou X, Han Y, Yang D, Liu Y, Fu J, Zheng S, He D, Zhou L, Zeng C (2014) Dopamine d(1)- like receptors suppress proliferation of vascular smooth muscle cell induced by insulin-like growth factor-1. Clin Exp Hypertens 36(3):140–147
Xiao-na Cai, Sa Shi, Hong-zhu Li, Li-na Wang, Li Zhang, Hong Li (2015) Effects and mechanisms of low concentration dopamine on hydrogen peroxide-induced apoptosis in cultured neonatal rat cardiomyocytes. Chin J Appl Physiol 31(1):67–71
Gorenne I, Kavurma M, Scott S, Bennett M (2006) Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res 72(1):9–17
Li H, Horke S, Förstermann U (2014) Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237(1):208–219
Gnanaguru G, Choi AR, Amarnani D, D’Amore PA (2016) Oxidized lipoprotein uptake through the CD36 receptor activates the NLRP3 inflammasome in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 57(11):4704–4712
Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M (2009) Dopamine, behavioral economics, and effort. Front Behav Neurosci 7(3):13
Cuevas S, Villar VA, Jose PA, Armando I (2013) Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci 14(9):17553–17572
Chugh G, Lokhandwala MF, Asghar M (2011) Oxidative stress alters renal D1 and AT1 receptor functions and increased blood pressure in old rat. Am J Physiol Renal Physiol 300(1):F133–F138
Brixius K, Schwinger RH, Hoyer F, Napp A, Renner R, Bölck B, Kümin A, Fischer U, Mehlhorn U, Werner S, Bloch W (2007) Isoform-specific downregulation of peroxiredoxin in human failing myocardium. Life Sci 81(10):823–831
Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, de Jong PE, Gans RO, Struck J, Schulte J, Hillege HL, van der Harst P, Peelen LM, Beulens JW, Stolk RP, Navis G, Bakker SJ (2012) Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J Am Heart Assoc 1(5):e002956
Ishii T (2015) Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radic Biol Med 88(Pt B):189–198
Kim H, Jung Y, Shin BS, Kim H, Song H, Bae SH, Rhee SG, Jeong W (2010) Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1and Nrf2. J Biol Chem 285(45):34419–34428
Isenovic ER, Kedees MH, Haidara MA, Trpkovic A, Mikhailidis DP, Marche P (2010) Involvement of ERK1/2 kinase in insulin-and thrombin-stimulated vascular smooth muscle cell proliferation. Angiology 61(4):357–364
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143(17):3050–3060
Acknowledgments
This work was supported by the National Natural Science Foundation of China Projects (No. 81270311), Heilongjiang Province Natural Science Foundation of China (No. D200880) and Natural Science foundation of Heilongjiang Education Department (No. 12521181).
Author information
Authors and Affiliations
Contributions
CX, HL supervised the study. HL, CX and SS participated in study design. HL, SS and JC contributed to the scientific discussion of the data. HL and JC performed the experiments.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have seen and approved the final manuscript and have taken due care to ensure the integrity of the work. The authors have declared that no conflict of interests exist.
Rights and permissions
About this article
Cite this article
Chen, J., Shi, S., Cai, X. et al. DR1 activation reduces the proliferation of vascular smooth muscle cells by JNK/c-Jun dependent increasing of Prx3. Mol Cell Biochem 440, 157–165 (2018). https://doi.org/10.1007/s11010-017-3164-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3164-0