Abstract
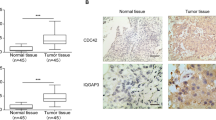
High expression of connexins was found in a variety of cancers, but their role is still controversial. We investigated whether connexin43 (Cx43) contributed to bladder carcinogenesis through MAPK activation. In this study, we found that Cx43 expression was significantly increased in bladder cancer tissues and cell line. Overexpression of Cx43 in bladder cancer 5637 cells increased cell proliferation, promoted cell cycle progression, and inhibited apoptosis. Western blot showed that JNK and ERK pathways were dramatically activated in Cx43-overexpressed cells. Conversely, knockdown of Cx43 inhibited cell proliferation by increasing apoptosis and causing cell cycle arrest, concomitant with inhibition of JNK and ERK signaling. In addition, JNK and ERK pathways were also activated in bladder cancer tissues. In conclusion, abnormal high expression and cytoplasmic localization of Cx43 contributed to bladder cancer. Inhibition of Cx43 activity could be a potential therapeutic strategy for preventing the progression of bladder cancer.




Similar content being viewed by others
References
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63:234–241. doi:10.1016/j.eururo.2012.07.033
Haddad AQ, Singla N, Gupta N, Raj GV, Sagalowsky AI, Margulis V, Lotan Y (2015) Association of distance to treatment facility on quality and survival outcomes after radical cystectomy for bladder cancer. Urology 85:876–882. doi:10.1016/j.urology.2014.12.024
Laird DW (2006) Life cycle of connexins in health and disease. Biochem J 394:527–543. doi:10.1042/BJ20051922
Goodenough DA, Paul DL (2009) Gap junctions. Cold Spring Harb Perspect Biol 1:a002576. doi:10.1101/cshperspect.a002576
Yeager M, Harris AL (2007) Gap junction channel structure in the early 21st century: facts and fantasies. Curr Opin Cell Biol 19:521–528. doi:10.1016/j.ceb.2007.09.001
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K (2005) Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10:459–469. doi:10.1007/s10495-005-1875-2
Wei CJ, Xu X, Lo CW (2004) Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 20:811–838. doi:10.1146/annurev.cellbio.19.111301.144309
Su V, Lau AF (2014) Connexins: mechanisms regulating protein levels and intercellular communication. FEBS Lett 588:1212–1220. doi:10.1016/j.febslet.2014.01.013
Solan JL, Lampe PD (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419:261–272. doi:10.1042/BJ20082319
Zhao W, Han HB, Zhang ZQ (2011) Suppression of lung cancer cell invasion and metastasis by connexin43 involves the secretion of follistatin-like 1 mediated via histone acetylation. Int J Biochem Cell Biol 43:1459–1468. doi:10.1016/j.biocel.2011.06.009
Tittarelli A, Guerrero I, Tempio F, Gleisner MA, Avalos I, Sabanegh S, Ortiz C, Michea L, Lopez MN, Mendoza-Naranjo A, Salazar-Onfray F (2015) Overexpression of connexin 43 reduces melanoma proliferative and metastatic capacity. Br J Cancer 113:259–267. doi:10.1038/bjc.2015.162
Ableser MJ, Penuela S, Lee J, Shao Q, Laird DW (2014) Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo. J Biol Chem 289:1592–1603. doi:10.1074/jbc.M113.507228
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2010) The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res 70:4222–4232. doi:10.1158/0008-5472.can-09-3281
Li Z, Zhou Z, Welch DR, Donahue HJ (2008) Expressing connexin 43 in breast cancer cells reduces their metastasis to lungs. Clin Exp Metastasis 25:893–901. doi:10.1007/s10585-008-9208-9
Danos K, Brauswetter D, Birtalan E, Pato A, Bencsik G, Krenacs T, Petak I, Tamas L (2015) The Potential Prognostic Value of Connexin 43 Expression in Head and Neck Squamous Cell Carcinomas. Appl Immunohistochem Mol Morphol. doi:10.1097/pai.0000000000000212
Han Y, Zhang PJ, Chen T, Yum SW, Pasha T, Furth EE (2011) Connexin43 Expression Increases in the Epithelium and Stroma along the Colonic Neoplastic Progression Pathway: Implications for Its Oncogenic Role. Gastroenterol Res Pract 2011:561719. doi:10.1155/2011/561719
Qin H, Shao Q, Curtis H, Galipeau J, Belliveau DJ, Wang T, Alaoui-Jamali MA, Laird DW (2002) Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J Biol Chem 277:29132–29138. doi:10.1074/jbc.M200797200
Stoletov K, Strnadel J, Zardouzian E, Momiyama M, Park FD, Kelber JA, Pizzo DP, Hoffman R, VandenBerg SR, Klemke RL (2013) Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci 126:904–913. doi:10.1242/jcs.112748
Corteggio A, Florio J, Roperto F, Borzacchiello G (2011) Expression of gap junction protein connexin 43 in bovine urinary bladder tumours. J Comp Pathol 144:86–90. doi:10.1016/j.jcpa.2010.05.002
Asamoto M, Takahashi S, Imaida K, Shirai T, Fukushima S (1994) Increased gap junctional intercellular communication capacity and connexin 43 and 26 expression in rat bladder carcinogenesis. Carcinogenesis 15:2163–2166
Loewenstein WRK, Y. (1966) Intercellular communication and the control of tissue growth :lack of communication between cancer cells. Nature
Poyet C, Buser L, Roudnicky F, Detmar M, Hermanns T, Mannhard D, Hohn A, Ruschoff J, Zhong Q, Sulser T, Moch H, Wild PJ (2015) Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer. J Clin Pathol 68:819–824. doi:10.1136/jclinpath-2015-202898
DavidJ. Panka MBA, andJames W. Mier (2006) Targeting the mitogen-activated protein kinase pathway in the treatment of malignant melanoma. Clin Cancer Res. doi:10.1158/1078-0432.CCR-05-2539
Milone MR, Pucci B, Bruzzese F, Carbone C, Piro G, Costantini S, Capone F, Leone A, Di Gennaro E, Caraglia M, Budillon A (2013) Acquired resistance to zoledronic acid and the parallel acquisition of an aggressive phenotype are mediated by p38-MAP kinase activation in prostate cancer cells. Cell Death Dis 4:e641. doi:10.1038/cddis.2013.165
Liang Z, Wu R, Xie W, Geng H, Zhao L, Xie C, Wu J, Geng S, Li X, Zhu M, Zhu W, Zhu J, Huang C, Ma X, Zhong C, Han H (2015) Curcumin Suppresses MAPK Pathways to Reverse Tobacco Smoke-induced Gastric Epithelial-Mesenchymal Transition in Mice. Phytother Res 29:1665–1671. doi:10.1002/ptr.5398
Sun X, Deng QF, Liang ZF, Zhang ZQ, Zhao L, Geng H, Xie DD, Wang Y, Yu DX, Zhong CY (2016) Curcumin reverses benzidine-induced cell proliferation by suppressing ERK1/2 pathway in human bladder cancer T24 cells. Exp Toxicol Pathol 68:215–222. doi:10.1016/j.etp.2015.12.003
Funding
This work was supported by grants from the scientific research youth fund project of affiliated hospital of Chengde medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Xiao-lin Ai and Qiang Chi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ai, Xl., Chi, Q., Qiu, Y. et al. Gap junction protein connexin43 deregulation contributes to bladder carcinogenesis via targeting MAPK pathway. Mol Cell Biochem 428, 109–118 (2017). https://doi.org/10.1007/s11010-016-2921-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2921-9