Abstract
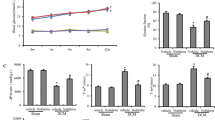
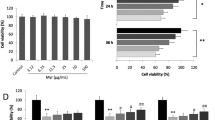
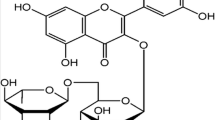
Apigenin is an important component of fruits and vegetables in human daily diets. Several cellular and animal models have been performed to demonstrate its anti-oxidant and anti-inflammatory bioactivities. However, the cardioprotective effects of apigenin in diabetic cardiomyopathy (DCM) remain unclear. In this study, we intended to explore the roles of apigenin in cardiac remodeling of DCM. Male C57BL/6 J mice were treated with streptozotocin (STZ, 50 mg/kg) for 5 consecutive days to induce DCM. The echocardiography and catheter-based measurements of hemodynamic parameters were performed to evaluate the cardiac function. Paraffin slices of harvested hearts were prepared for histological pathological analysis and TUNEL assay. Oxidative assay kits were used to detect Glutathione Peroxidase (GPx), Lipid Peroxidation Malondialdehyde (MDA), and Superoxide Dismutase (SOD). Western blot and real-time PCR were used for accessing the expressions of protein and mRNA. Diabetes mellitus exacerbated the cardiac dysfunction, fibrosis, and overaccumulation of 4-hydroxynonenal accompanying with down-regulation of Bcl2, GPx, and SOD, up-regulation of MDA, cleaved caspase3, and pro-apoptotic protein Bax, and contribution to the translocation of NF-κB. All these pathological changes could be effectively blunted by treatment of apigenin in vivo. Finally, H9c2 treated with high glucose or apigenin was used for further investigation of these effects in vitro; what is more, we also compared the effects between apigenin and Resveratrol in in vitro experiments. Our experiments have demonstrated that apigenin may be a potential drug for diabetic patients suffering from DCM.









Similar content being viewed by others
References
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12:144–153
Aroor AR, Mandavia CH, Sowers JR (2012) Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clinics 8:609–617
Wong AK, AlZadjali MA, Choy AM, Lang CC (2008) Insulin resistance: a potential new target for therapy in patients with heart failure. Cardiovasc Ther 26:203–213
Lefort EC, Blay J (2013) Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res 57:126–144
Shukla S, Gupta S (2010) Apigenin: a promising molecule for cancer prevention. Pharm Res 27:962–978
Patel D, Shukla S, Gupta S (2007) Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol 30:233–245
Liu-Smith F, Meyskens F (2016) Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res 60:1264–1274
Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, Wang TS, Chen HW (2013) Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxico 87:167–178
Ohno M, Shibata C, Kishikawa T, Yoshikawa T, Takata A, Kojima K, Akanuma M, Kang YJ, Yoshida H, Otsuka M, Koike K (2013) The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci Rep 3:2553
Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN (2013) Flavonoid apigenin is an inhibitor of the NAD + ase CD38: implications for cellular NAD + metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62:1084–1093
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Haskó G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher PC (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56:2115–2125
Bai D, Zhang Y, Shen M, Sun Y, Xia Q, Zhang Y, Liu X, Wang H, Yuan L (2016) Hyperglycemia and hyperlipidemia blunts the Insulin-Inpp5f negative feedback loop in the diabetic heart. Sci Rep 6:22068
Testai L, Martelli A, Cristofaro M, Breschi MC, Calderone V (2013) Cardioprotective effects of different flavonoids against myocardial ischaemia/ reperfusion injury in Langendorff-perfused rat hearts. J Pharm Pharmacol 65: 750–756
Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L (2013) Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol 57:82–95
Liao HH, Zhang N, Feng H, Zhang N, Ma ZG, Yang Z, Yuan Y, Bian ZY, Tang QZ (2015) Oleanolic acid alleviated pressure overload-induced cardiac remodeling. Mol Cell Biochem 409:145–154
LaRocca TJ, Fabris F, Chen J, Benhayon D, Zhang S, McCollum L, Schecter AD, Cheung JY, Sobie EA, Hajjar RJ, Lebeche D (2012) Na+/Ca2 + exchanger-1 protects against systolic failure in the Akitains2 model of diabetic cardiomyopathy via a CXCR4/NF-kappaB pathway. Am J Physiol Heart Circ Physiol 303:H353–H367
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Sung MM, Hamza SM, Dyck JRB (2015) Myocardial metabolism in diabetic cardiomyopathy: potential therapeutic targets. Antioxidants Redox Signal 22:1606–1630
Shukla S, Kanwal R, Shankar E, Datt M, Chance MR, Fu P, MacLennan GT, Gupta S (2015) Apigenin blocks IKKalpha activation and suppresses prostate cancer progression. Oncotarget 6:31216–31232
Beckman JA, Creager MA (2016) Vascular complications of Diabetes. Circ Res 118:1771–1785
Saunders J, Mathewkutty S, Drazner MH, McGuire DK (2008) Cardiomyopathy in type 2 diabetes: update on pathophysiological mechanisms. Herz 33:184–190
Standl E, Schnell O, McGuire DK (2016) Heart failure considerations of antihyperglycemic medications for type 2 diabetes. Circ Res 118:1830–1843
Seferovic PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36:1718–1727
Arango D, Diosa-Toro M, Rojas-Hernandez LS, Cooperstone JL, Schwartz SJ, Mo X, Jiang J, Schmittgen TD Doseff AI (2015) Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol Nutr Food Res 59:763–772
Cao HH, Chu JH, Kwan HY, Su T, Yu H, Cheng CY, Fu XQ, Guo H, Li T, Tse AK, Chou GX, Mo HB, Yu ZL 2016 Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci Rep 6:21731
Arango D, Morohashi K, Yilmaz A, Kuramochi K, Parihar A, Brahimaj B, Grotewold E, Doseff AI (2013) Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc Natl Acad Sci USA 110:E2153–2162
Clark JL, Zahradka P, Taylor CG (2015) Efficacy of flavonoids in the management of high blood pressure. Nutr Rev 73:799–822
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81270303, 81470516,81470402), Hubei Province’s Outstanding Medical Academic Leader program, and the Fundamental Research Funds for the Central Universities of China (No. 2015301020202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Huang-Jun Liu and Yun-Lin Fan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, HJ., Fan, YL., Liao, HH. et al. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol Cell Biochem 428, 9–21 (2017). https://doi.org/10.1007/s11010-016-2913-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2913-9