Abstract
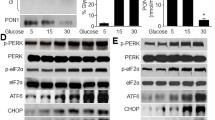
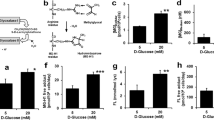
Type 2 Diabetes Mellitus is a worldwide epidemic, and its atherosclerotic complications produce morbidity and mortality in affected patients. It is known that the vascular cell adhesion molecule-1 (VCAM-1) levels are increased in the sera of diabetic patients. Our aim was to investigate the impact of the endoplasmic reticulum stress (ERS) in VCAM-1 expression and secretion in human endothelial cells (HEC) exposed to glycated low-density lipoproteins (gLDL). The results showed that 24 h incubation of HEC with gLDL induces (i) stimulation of VCAM-1 expression and secretion, determining increased monocyte adhesion to HEC; (ii) RAGE up-regulation and free cholesterol loading; (iii) ERS activation (increased eIF2α phosphorylation and CHOP mRNA levels, and decreased GRP78 protein expression); and (iv) oxidative stress [increased levels of reactive oxygen species (ROS) and glutamate cysteine ligase catalytic unit gene expression]. Treatment of gLDL-exposed HEC with ERS inhibitors, salubrinal (Sal) and sodium phenylbutyrate (PBA), decreased intracellular ROS. Incubation of gLDL-exposed cells with the anti-oxidant N-acetyl-cysteine (NAC) reduced ERS, revealed by decreased eIF2α phosphorylation and CHOP gene expression and increased GRP78 expression, thus validating the interconnection between ERS and oxidative stress. Sal, PBA, NAC and inhibitors of p38 MAP kinase and NF-kB induced the decrease of VCAM-1 expression and of the ensuing monocyte adhesion induced by gLDL. In conclusion, in HEC, gLDL stimulate the expression of cellular VCAM-1, the secretion of soluble VCAM-1, and the adhesion of monocytes through mechanisms involving p38 MAP kinase and NF-kB signalling pathways activated by RAGE, ERS and oxidative stress, thus contributing to diabetic atherosclerosis.






Similar content being viewed by others
References
Basha B, Samuel SM, Triggle CR, Ding H (2012) Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress? Exp Diabetes Res. doi:10.1155/2012/481840
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114(6):597–605. doi:10.1161/CIRCULATIONAHA.106.621854
Negre-Salvayre A, Salvayre R, Auge N, Pamplona R, Portero-Otin M (2009) Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal 11(12):3071–3109. doi:10.1089/ARS.2009.2484
Vlassara H, Striker GE (2011) AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 7(9):526–539. doi:10.1038/nrendo.2011.74
Younis N, Charlton-Menys V, Sharma R, Soran H, Durrington PN (2009) Glycation of LDL in non-diabetic people: small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 202(1):162–168. doi:10.1016/j.atherosclerosis.2008.04.036
Sima A, Stancu C (2002) Modified lipoproteins accumulate in human coronary atheroma. J Cell Mol Med 6(1):110–111. doi:10.1111/j.1582-4934.2002.tb00316.x
Mestas J, Ley K (2008) Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18(6):228–232. doi:10.1016/j.tcm.2008.11.004
Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD (2000) Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes 49(3):485–491
Sozer V, Himmetoglu S, Korkmaz GG, Kaya S, Aydin S, Yumuk V, Hatemi H, Uzun H (2014) Paraoxonase, oxidized low density lipoprotein, monocyte chemoattractant protein-1 and adhesion molecules are associated with macrovascular complications in patients with type 2 diabetes mellitus. Minerva Med 105(3):237–244
Toma L, Stancu CS, Sanda GM, Sima AV (2011) Anti-oxidant and anti-inflammatory mechanisms of amlodipine action to improve endothelial cell dysfunction induced by irreversibly glycated LDL. Biochem Biophys Res Commun 411(1):202–207. doi:10.1016/j.bbrc.2011.06.137
Zhao R, Ren S, Moghadasain MH, Rempel JD, Shen GX (2014) Involvement of fibrinolytic regulators in adhesion of monocytes to vascular endothelial cells induced by glycated LDL and to aorta from diabetic mice. J Leukoc Biol 95(6):941–949. doi:10.1189/jlb.0513262
Toma L, Stancu CS, Botez GM, Sima AV, Simionescu M (2009) Irreversibly glycated LDL induce oxidative and inflammatory state in human endothelial cells; added effect of high glucose. Biochem Biophys Res Commun 390(3):877–882. doi:10.1016/j.bbrc.2009.10.066
Sima AV, Botez GM, Stancu CS, Manea A, Raicu M, Simionescu M (2010) Effect of irreversibly glycated LDL in human vascular smooth muscle cells: lipid loading, oxidative and inflammatory stress. J Cell Mol Med 14(12):2790–2802. doi:10.1111/j.1582-4934.2009.00933.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Manduteanu I, Pirvulescu M, Gan AM, Stan D, Simion V, Dragomir E, Calin M, Manea A, Simionescu M (2010) Similar effects of resistin and high glucose on P-selectin and fractalkine expression and monocyte adhesion in human endothelial cells. Biochem Biophys Res Commun 391(3):1443–1448. doi:10.1016/j.bbrc.2009.12.089
Deleanu M, Sanda GM, Stancu CS, Popa ME, Sima AV (2016) Profiles of fatty acids and the main lipid peroxidation products of human atherogenic low density lipoproteins. Rev Chim (Bucharest) 67(1):8–12
Tames FJ, Mackness MI, Arrol S, Laing I, Durrington PN (1992) Non-enzymatic glycation of apolipoprotein B in the sera of diabetic and non-diabetic subjects. Atherosclerosis 93(3):237–244
Williams KJ, Tabas I (1998) The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol 9(5):471–474
Stancu CS, Toma L, Sima AV (2012) Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res 349(2):433–446. doi:10.1007/s00441-012-1437-1
Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S (2002) Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 22(10):1649–1654
Stirban A, Gawlowski T, Roden M (2014) Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol Metab 3(2):94–108. doi:10.1016/j.molmet.2013.11.006
Zarubin T, Han J (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15(1):11–18. doi:10.1038/sj.cr.7290257
Cybulsky MI, Fries JW, Williams AJ, Sultan P, Eddy R, Byers M, Shows T, Gimbrone MA Jr, Collins T (1991) Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci USA 88(17):7859–7863
Zhao R, Xie X, Le K, Li W, Moghadasian MH, Beta T, Shen GX (2015) Endoplasmic reticulum stress in diabetic mouse or glycated LDL-treated endothelial cells: protective effect of Saskatoon berry powder and cyanidin glycans. J Nutr Biochem 26(11):1248–1253. doi:10.1016/j.jnutbio.2015.05.015
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318(5852):944–949. doi:10.1126/science.1146361
Yamagishi N, Ueda T, Mori A, Saito Y, Hatayama T (2012) Decreased expression of endoplasmic reticulum chaperone GRP78 in liver of diabetic mice. Biochem Biophys Res Commun 417(1):364–370. doi:10.1016/j.bbrc.2011.11.118
Hernandez Vera R, Vilahur G, Ferrer-Lorente R, Pena E, Badimon L (2012) Platelets derived from the bone marrow of diabetic animals show dysregulated endoplasmic reticulum stress proteins that contribute to increased thrombosis. Arterioscler Thromb Vasc Biol 32(9):2141–2148. doi:10.1161/ATVBAHA.112.255281
Wong DP, Chu JM, Hung VK, Lee DK, Cheng CH, Yung KK, Yue KK (2013) Modulation of endoplasmic reticulum chaperone GRP78 by high glucose in hippocampus of streptozotocin-induced diabetic mice and C6 astrocytic cells. Neurochem Int 63(6):551–560. doi:10.1016/j.neuint.2013.09.010
Wu HL, Li YH, Lin YH, Wang R, Li YB, Tie L, Song QL, Guo DA, Yu HM, Li XJ (2009) Salvianolic acid B protects human endothelial cells from oxidative stress damage: a possible protective role of glucose-regulated protein 78 induction. Cardiovasc Res 81(1):148–158. doi:10.1093/cvr/cvn262
Xue J, Wei J, Dong X, Zhu C, Li Y, Song A, Liu Z (2013) ABCG1 deficiency promotes endothelial apoptosis by endoplasmic reticulum stress-dependent pathway. J Physiol Sci: JPS 63(6):435–444. doi:10.1007/s12576-013-0281-8
Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H (2001) Cd36, a member of the class b scavenger receptor family, as a receptor for advanced glycation end products. J Biol Chem 276(5):3195–3202. doi:10.1074/jbc.M006545200
Luo Y, Li SJ, Yang J, Qiu YZ, Chen FP (2013) HMGB1 induces an inflammatory response in endothelial cells via the RAGE-dependent endoplasmic reticulum stress pathway. Biochem Biophys Res Commun 438(4):732–738. doi:10.1016/j.bbrc.2013.07.098
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol. doi:10.1155/2012/936486
Sheikh-Ali M, Sultan S, Alamir AR, Haas MJ, Mooradian AD (2010) Effects of antioxidants on glucose-induced oxidative stress and endoplasmic reticulum stress in endothelial cells. Diabetes Res Clin Pract 87(2):161–166. doi:10.1016/j.diabres.2009.10.023
Shen L, Sevanian A (2001) OxLDL induces macrophage gamma-GCS-HS protein expression: a role for oxLDL-associated lipid hydroperoxide in GSH synthesis. J Lipid Res 42(5):813–823
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A (2009) Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89(1):27–71. doi:10.1152/physrev.00014.2008
Wen Y, Skidmore JC, Porter-Turner MM, Rea CA, Khokher MA, Singh BM (2002) Relationship of glycation, antioxidant status and oxidative stress to vascular endothelial damage in diabetes. Diabetes Obes Metab 4(5):305–308. doi:10.1046/j.1463-1326.2002.00212.x
Norman MU, James WG, Hickey MJ (2008) Differential roles of ICAM-1 and VCAM-1 in leukocyte-endothelial cell interactions in skin and brain of MRL/faslpr mice. J Leukoc Biol 84(1):68–76. doi:10.1189/jlb.1107796
Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K (1999) Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res 84(11):1237–1244
Wang YI, Bettaieb A, Sun C, DeVerse JS, Radecke CE, Mathew S, Edwards CM, Haj FG, Passerini AG, Simon SI (2013) Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS One 8(10):e78322. doi:10.1371/journal.pone.0078322
Zhao W, Wu C, Chen X (2015) Cryptotanshinone inhibits oxidized LDL-induced adhesion molecule expression via ROS dependent NF-kappaB pathways. Cell Adhes Migr. doi:10.1080/19336918.2015.1119361
Kawanami D, Matoba K, Okada R, Tsukamoto M, Kinoshita J, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K (2013) Fasudil inhibits ER stress-induced VCAM-1 expression by modulating unfolded protein response in endothelial cells. Biochem Biophys Res Commun 435(2):171–175. doi:10.1016/j.bbrc.2013.04.091
Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. doi:10.3389/fncel.2014.00213
Darling NJ (1843) Cook SJ (2014) The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta 10:2150–2163. doi:10.1016/j.bbamcr.2014.01.009
Acknowledgments
The authors thank Ms. Daniela Rogoz and Ms. Cristina Dobre for their skilful technical assistance. This work was supported by the Romanian Academy, the Romanian Ministry of National Education PN-II-PT-PCCA-2011-3.1-0184 project (Grant Number PCCA-127/2012) and PN-II-RU-TE-2014-4-0506 project (Grant Number TE 11/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
This article is dedicated to the 150th Anniversary of the Romanian Academy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Toma, L., Sanda, G.M., Deleanu, M. et al. Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress. Mol Cell Biochem 417, 169–179 (2016). https://doi.org/10.1007/s11010-016-2724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2724-z