Abstract
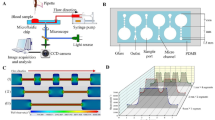
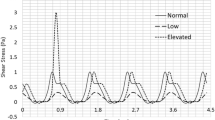
The purpose of this study was to investigate the influence of non-physiological high shear stress on activation and shedding of platelet GP IIb/IIIa receptors. The healthy donor blood was exposed to three levels of high shear stresses (25, 75, 125 Pa) from the physiological to non-physiological status with three short exposure time (0.05, 0.5, 1.5 s), created by a specific blood shearing system. The activation and shedding of the platelet GPIIb/IIIa were analyzed using flow cytometry and enzyme-linked immunosorbent assay. In addition, platelet P-selectin expression of sheared blood, which is a marker for activated platelets, was also analyzed. The results from the present study showed that the number of activated platelets, as indicated by the surface GPIIb/IIIa activation and P-selectin expression, increased with increasing the shear stress level and exposure time. However, the mean fluorescence of GPIIb/IIIa on the platelet surface, decreased with increasing the shear stress level and exposure time. The reduction of GPIIb/IIIa on the platelet surface was further proved by the reduction of further activated platelet GPIIb/IIIa surface expression induced by ADP and the increase in GPIIb/IIIa concentration in microparticle-free plasma with increasing the applied shear stress and exposure time. It is clear that non-physiological shear stress induce a paradoxical phenomenon, in which both activation and shedding of the GPIIb/IIIa on the platelet surface occur simultaneously. This study may offer a new perspective to explain the reason of both increased thrombosis and bleeding events in patients implanted with high shear blood-contacting medical devices.





Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL et al (2014) Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129:28–292
National Kidney Foundation (2012) The facts about chronic kidney disease. National Kidney Foundation, New York
Eckman PM, John R (2012) Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 125:3038–3047
Boyle AJ, Russell SD, Teuteberg JJ et al (2009) Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transpl 28:881–887
Bruckner BA, DiBardino DJ, Ning Q, Adeboygeun A, Mahmoud K, Valdes J et al (2009) High incidence of thromboembolic events in left ventricular assist device patients treated with recombinant activated factor VII. J Heart Lung Transpl 28:785–790
John R, Kamdar F, Liao K et al (2008) Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg 136:1318–1323
Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ (2012) A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng 134(8):081002
Alemu Y, Bluestein D (2007) Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif Organs 31(9):677–688
Morbiducci U, Ponzini R, Nobili M, Massai D, Montevecchi FM, Bluestein D, Redaelli A (2009) Blood damage safety of prosthetic heart valves: shear-induced platelet activation and local flow dynamics: a fluid-structure interaction approach. J Biomech 42(12):1952–1960
Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D (2008) Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J 54(1):64–72
Song X, Throckmorton AL, Wood HG, Antaki JF, Olsen DB (2004) Quantitative evaluation of blood damage in a centrifugal VAD by computational fluid dynamics. J Fluids Eng 126:410–418
Bluestein D (2004) Research approaches for studying flow-induced thromboembolic complications in blood recirculating devices. Expert Rev Med Devices 1(1):65–80
Deutsch S, Tarbell JM, Manning KB, Rosenberg G, Fontaine AA (2006) Experimental fluid mechanics of pulsatile artificial blood pumps. Annu Rev Fluid Mech 38:65–86
Rivera J, Lozano ML, Navarro-Núñez L, Vicente V (2009) Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 94(5):700–711
Li Z, Delaney MK, O’Brien KA, Du X (2010) Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 30(12):2341–2349
John R, Panch S, Hrabe J, Wei P, Solovey A, Joyce L, Hebbel R (2009) Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg 88(4):1171–1179
Steinlechner B, Dworschak M, Birkenberg B, Duris M, Zeidler P, Fischer H, Milosevic L, Wieselthaler G, Wolner E, Quehenberger P, Jilma B (2009) Platelet dysfunction in outpatients with left ventricular assist devices. Ann Thorac Surg 87(1):131–137
Avrahami I, Rosenfeld M, Einav S (2006) The hemodynamics of the Berlin pulsatile VAD and the role of its MHV configuration. Ann Biomed Eng 34:1373–1388
Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE (1996) Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 88(3):907–914
Phillips DR, Charo IF, Parise LV, Fitzgerald LA (1988) The platelet membrane glycoprotein IIb-IIIa complex. Blood 71(4):831–843
Reddy KB, Smith DM, Plow EF (2008) Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J Cell Sci 15:1211641–1211648
Canobbio I, Balduini C, Torti M (2004) Signalling through the platelet glycoprotein Ib-V-IX complex. Cell Signal 16(12):1329–1344
Cheng H, Yan R, Li S et al (2009) Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibα shedding. Am J Physiol Heart Circ Physiol 297:H2128–H2135
Hu J, Mondal NK, Sorensen EN et al (2014) Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transpl 33:71–79
Al-Tamimi M, Tan CW, Qiao J et al (2012) Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood 119:4311–4320
Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP (2007) Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 109:566–776
Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL (1996) Platelets and shear stress. Blood 88(5):1525–1541
Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S (1996) High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 88:3456–3464
Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH, Jackson SP (2002) Distinct glycoprotein Ib/V/IX and integrin alpha(IIb)beta(3)-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem 277:2965–2972
Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ (2004) Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood 103:3403–3411
Wenger RK, Lukasiewicz H, Mikuta BS, Niewiarowski S, Edmunds LH Jr (1989) Loss of platelet fibrinogen receptors during clinical cardiopulmonary bypass. J Thorac Cardiovasc Surg 97(2):235–239
Du X, Saido TC, Tsubuki S, Indig FE, Williams MJ, Ginsberg MH (1995) Calpain cleavage of the cytoplasmic domain of the integrin β3 subunit. J Biol Chem 270:26146–26151
Pfaff M, Du X, Ginsberg MH (1999) Calpain cleavage of integrin βa cytoplasmic domains. FEBS Lett 460:17–22
Zhang T, Taskin ME, Fang HB et al (2011) Study of flow-induced hemolysis using novel Couette-type blood-shearing devices. Artif Organs 35:1180–1186
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282(21):2035–2042
Taskin ME, Fraser KH, Zhang T, Wu C, Griffith BP, Wu ZJ (2012) Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO J 58:363–372
Acknowledgments
This work was partially supported by the National Institutes of Health (Grant numbers: R01 HL 088100, R01 HL124170) and the International Postdoctoral Exchange Fellowship Program (No. 20130028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflicts of interests.
Additional information
Zengsheng Chen and Nandan K. Mondal have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Z., Mondal, N.K., Ding, J. et al. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol Cell Biochem 409, 93–101 (2015). https://doi.org/10.1007/s11010-015-2515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2515-y