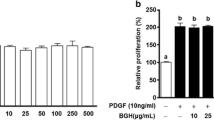
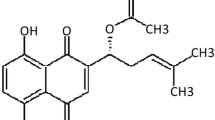
Abstract
Bioflavonoids are known to induce cardioprotective effects by inhibiting vascular smooth muscle cell (VSMC) proliferation and migration. Kaempferol has been shown to inhibit VSMC proliferation. However, little is known about the effect of kaempferol on VSMC migration and the underlying molecular mechanisms. Our studies provide the first evidence that kaempferol inhibits VSMC migration by modulating the BMP4 signaling pathway and microRNA expression levels. Kaempferol activates the BMP signaling pathway, induces miR-21 expression and downregulates DOCK4, 5, and 7, leading to inhibition of cell migration. Moreover, kaempferol antagonizes the PDGF-mediated pro-migratory effect. Therefore, our study uncovers a novel regulatory mechanism of VSMC migration by kaempferol and suggests that miRNA modulation by kaempferol is a potential therapy for cardiovascular diseases.




Similar content being viewed by others
References
Hertog MG, Feskens EJ, Kromhout D (1997) Antioxidant flavonols and coronary heart disease risk. Lancet 349:699. doi:10.1016/S0140-6736(05)60135-3
Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M (2011) A review on the dietary flavonoid kaempferol. Mini Rev Med Chem 11:298–344
Wu WB, Hung DK, Chang FW, Ong ET, Chen BH (2012) Anti-inflammatory and anti-angiogenic effects of flavonoids isolated from Lycium barbarum Linnaeus on human umbilical vein endothelial cells. Food Funct 3:1068–1081. doi:10.1039/c2fo30051f
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Kong L, Luo C, Li X, Zhou Y, He H (2013) The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis 12:115. doi:10.1186/1476-511X-12-115
Chen SS, Michael A, Butler-Manuel SA (2012) Advances in the treatment of ovarian cancer: a potential role of antiinflammatory phytochemicals. Discov Med 13:7–17
Kim SY, Jin YR, Lim Y, Kim JH, Cho MR, Hong JT, Yoo HS, Yun YP (2005) Inhibition of PDGF beta-receptor tyrosine phosphorylation and its downstream intracellular signal transduction in rat aortic vascular smooth muscle cells by kaempferol. Planta Med 71:599–603. doi:10.1055/s-2005-871263
Nepal M, Li L, Cho HK, Park JK, Soh Y (2013) Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling pathway. Food Chem Toxicol 62:238–245. doi:10.1016/j.fct.2013.08.034
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801. doi:10.1152/physrev.00041.2003
ten Dijke P, Arthur HM (2007) Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol 8:857–869
Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A (2010) Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J 29:559–573. doi:10.1038/emboj.2009.370
Kang H, Hata A (2012) MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol 19:224–231
Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, Lieberman J, Van Aelst L, Lagna G, Hata A (2011) Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem 287:3976–3986
Kang H, Louie J, Weisman A, Sheu-Gruttadauria J, Davis-Dusenbery BN, Lagna G, Hata A (2012) Inhibition of MicroRNA-302 (miR-302) by bone morphogenetic protein 4 (BMP4) facilitates the BMP signaling pathway. J Biol Chem 287:38656–38664
Kim S, Hata A, Kang H (2014) Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem 115:889–895. doi:10.1002/jcb.24730
Kim S, Kang H (2013) miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep 46:550–554
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56–61
Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ (1999) Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension 33:177–182
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD (2008) BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 14:1363–1369. doi:10.1038/nm.1888
Corella D, Ordovas JM (2014) How does the Mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. BioEssays 36:526–537. doi:10.1002/bies.201300180
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75:487–517
Altamemi I, Murphy EA, Catroppo JF, Zumbrun EE, Zhang J, McClellan JL, Singh UP, Nagarkatti PS, Nagarkatti M (2014) Role of microRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. J Pharmacol Exp Ther 350:99–109. doi:10.1124/jpet.114.213306
Davalos A, Fernandez-Hernando C (2013) From evolution to revolution: miRNAs as pharmacological targets for modulating cholesterol efflux and reverse cholesterol transport. Pharmacol Res 75:60–72. doi:10.1016/j.phrs.2013.02.005
Acknowledgments
This work was supported by the Incheon National University Research Grant in 2014.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2015_2464_MOESM1_ESM.eps
Supplementary material 1 (EPS 2057 kb). Supplemental Fig. 1. Anti-migratory function of kaempferol. PASMCs were treated with DMSO (MOCK), 50 μM kaempferol or 50 μM equol. After scratch wounds were generated, they were photographed over 24 h
11010_2015_2464_MOESM2_ESM.eps
Supplementary material 2 (EPS 1197 kb). Supplemental Fig. 2. Time dependent effects of kaempferol on the Smad1/5 phosphorylation. PASMCs were treated with 50 μM kaempferol for the indicated time. Total cell lysates were subjected to immunoblotting with anti-phospho-Smad1/5 (pSmad1/5) or β-actin antibodies
11010_2015_2464_MOESM3_ESM.eps
Supplementary material 3 (EPS 18478 kb). Supplemental Fig. 3. BMP signaling activity and kaempferol function. A PASMCs were treated with DMSO (MOCK) or 50 μM kaempferol for 24 h. mRNA levels of the indicated genes relative to GAPDH were measured using qRT-PCR. B. PASMCs treated with DMSO (MOCK) or 50 μM kaempferol were subjected to the scratch wound assay in the presence or absence of 2.5 μM of LDN193189. After scratch wounds were generated, they were photographed over 24 h
Rights and permissions
About this article
Cite this article
Kim, K., Kim, S., Moh, S.H. et al. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression. Mol Cell Biochem 407, 143–149 (2015). https://doi.org/10.1007/s11010-015-2464-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2464-5