Abstract
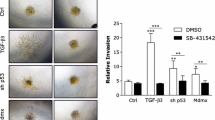
Tumor metastasis is the primary cause of mortality in most cancer patients. Before disassociation from the tumors, most of malignant tumor cells undergo the epithelial–mesenchymal transition to break away from the adhesions between the cells and the surrounding extracellular matrix. Recently, activating enhancer-binding protein (AP4) has been shown to be a mediator of EMT in colorectal cancer and high level of AP4 correlates with poor prognosis in cancer patients. It has been found that AP4 upregulates the genes involved in EMT and cell proliferation in colorectal cancer cells and that the aggressive human breast cancer cells MDA-MB-231 are highly metastatic. Therefore, we tested the hypothesis that AP4 may also affect cell migration and EMT in this cell type. Three different assays, including the wound-healing assay, the Boyden chamber assay, and the cell tracking assay, were employed to confirm that AP4 activated both cell migration and invasion. Immunofluorescence staining and Western blot analysis revealed that the cells underwent EMT when AP4 was upregulated. In contrast, overexpression of dominant-negative AP4, lacking the DNA-binding domain, inactivated the DNA-binding ability of endogenous AP4 and led to lower cell motility. Furthermore, we found that AP4 enhanced p53 expression at both transcriptional and translational levels. Knockdown of p53 by siRNA significantly diminished the activation of cell migration by AP4, indicating that AP4 can regulate cell migration via the activity of p53.








Similar content being viewed by others
Abbreviations
- AP4:
-
Activating enhancer-binding-4
- DN-AP4:
-
Dominant negative AP4
- bHLH:
-
Basic helix-loop-helix
- SV40:
-
Simian virus 40
- MMP-9:
-
Matrix metalloproteinase-9
- IGFBP-2:
-
Insulin-like growth factor-binding protein 2
- mdm2 :
-
Murine double minute clone 2
- EMT:
-
Epithelial–mesenchymal transition
- MET:
-
Mesenchymal–epithelial transition
- DOX:
-
Doxycycline
References
Klein CA (2008) Cancer. The metastasis cascade. Science 321:1785–1787
Meera S (2009) Metastasis: a malignant mutation. Nat Rev Cancer 9:233
Chantal VS, Johan B, Paul NS, Fred CS, Reidar G, Hanneke S, Ger JP, Johannes HK, Wilbert CB (2013) αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer 13:128
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, Zhang J, Ding P, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y (2010) R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 70:1544–1554
Mermod N, Williams TJ, Tjian R (1988) Enhancer binding factors AP4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature 332:557–561
Edmondson DG, Olson EN (1989) A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev 3:628–640
Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, Weintraub H (1989) MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 58:823–831
Ludwig SR, Habera LF, Dellaporta SL, Wessler SR (1989) Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA 86:7092–7096
Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB (1989) Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58:537–544
Murre C, McCaw PS, Baltimore D (1989) A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56:777–783
Kim MY, Jeong BC, Lee JH, Kee HJ, Kook H, Kim NS, Kim YH, Kim JK, Ahn KY, KK Kim (2006) A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc Natl Acad Sci USA 103:13074–13079
Imai K, Okamoto T (2006) Transcriptional repression of human immunodeficiency virus type 1 by AP4. J Biol Chem 281:12495–12505
Tsujimoto K, Ono T, Sato M, Nishida T, Oguma T, Tadakuma T (2005) Regulation of the expression of caspase-9 by the transcription factor activator protein-4 in glucocorticoid-induced apoptosis. J Biol Chem 280:27638–27644
Cao J, Tang M, Li WL, Xie J, Du H, Tang WB, Wang H, Chen XW, Xiao H, Li Y (2009) Up regulation of activator protein-4 in human colorectal cancer with metastasis. Int J Surg Pathol 17:16–21
Wang R, Zhang YW, Zhang X, Liu R, Zhang X, Hong S, Xia K, Xia J, Zhang Z, Xu H (2006) Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J 20:1275–1277
Glahder JA, Hansen CN, Vinther J, Madsen BS, Norrild B (2003) A promoter within the E6 ORF of human papillomavirus type 16 contributes to the expression of the E7 oncoprotein from a monocistronic mRNA. J Gen Virol 84:3429–3441
Jung P, Menssen A, Mayr D, Hermeking H (2008) AP4 encodes a c-MYC-inducible repressor of p21. Proc Natl Acad Sci USA 105:15046–15051
Lin TX, Huang J, Xu KW, Cai QQ, Li SY (2006) Identification of transcription factor AP4 as the regulator that upregulates the expression of l-plastin in hormone-independent prostate cancer. J Sun Yat-Sen Univ (Med Sci) 6:19–23
Amin S, Kumar A, Nilchi L, Wright K, Kozlowski M (2011) Breast cancer cells proliferation is regulated by tyrosine phosphatase SHP1 through c-jun n-terminal kinase and cooperative induction of RFX-1 and AP4 transcription factors. Mol Cancer Res 9:1112–1115
Buechler S (2009) Low expression of a few genes indicates good prognosis in estrogen receptor positive breast cancer. BMC Cancer 9:243
Ku WC, Chiu SK, Chen YJ, Huang HH, Wu WG, Chen YJ (2009) Complementary quantitative proteomics reveals that transcription factor AP4 mediates E-box-dependent complex formation for transcriptional repression of HDM2. Mol Cell Proteomics 8:2034–2050
Badinga L, Song S, Simmen RC, Simmen FA (1998) A distal regulatory region of the insulin-like growth factor binding protein-2 (IGFBP-2) gene interacts with the basic helix-loop-helix transcription factor, AP4. Endocrine 8:281–289
Jackstadt R, Röh S, Neumann J, Jung P, Hoffmann R, Horst D, Berens C, Bornkamm GW, Kirchner T, Menssen A, Hermeking H (2013) AP4 is a mediator of epithelial–mesenchymal transition and metastasis in colorectal cancer. J Exp Med 210:1331–1350
Hu BS, Zhao G, Yu HF, Chen K, Dong JH, Tan JW (2013) High expression of AP4 predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. Tumor Biol 34:271–276
Lauréline R, Gilles G, Pierre R (2006) Control of cell migration: a tumour suppressor function for p53? Biol Cell 98:141–152
Epstein CB, Attiyeh EF, Hobson DA, Silver AL, Broach JR, Levine AJ (1998) P53 mutations isolated in yeast based on loss of transcription factor activity: similarities and differences from p53 mutations detected in humans. Oncogene 16:2115–2122
Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH (2009) Mutant p53 drives invasion by promoting integrin recycling. Cell 139:1327–1341
Zhou X, Vink M, Klaver B, Berkhout B, Das AT (2006) Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther 13:1382–1390
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Yang SY, Zhang JJ, Huang XY (2009) Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 15:124–134
Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY (2006) The g protein g alpha(13) is required for growth factor-induced cell migration. Dev Cell 10:707–718
Yang S, Huang XY (2005) Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem 280:27130–27137
Kleinman HK (2001) Preparation of basement membrane components from EHS tumors. Curr Protoc Cell Biol. doi:10.1002/0471143030.cb1002s00
Freshney RI (1987) Culture of animal cells: a manual of basic technique. Alan R Liss, Inc., New York, p 117
Pfrepper KI, Flugel RM (2005) Molecular characterization of proteolytic processing of the gap proteins of human spumaretrovirus. Methods Mol Biol 304:435–444
Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A (2011) Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci 31(1):184–192
Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J (2011) The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J 25(1):35–44
Zhang X, Wong SM (2009) Hibiscus chlorotic ringspot virus upregulates plant sulfite oxidase transcripts and increases sulfate levels in kenaf (Hibiscus cannabinus L.). J Gen Virol 90:3042–3050
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25:402–408
Kogan SI, Tabach Y, Buganim Y, Molchadsky A, Solomon H, Madar S, Kamer I, Stambolsky P, Shelly A, Goldfinger N, Valsesia WS, Puisieux A, Zundelevich A, Galyam EN, Avivi C, Barshack I, Brait M, Sidransky D, Domany E, Rotter V (2011) Mutant p53(R175H) upregulates Twist1 expression and promotes epithelial–mesenchymal transition in immortalized prostate cells. Cell Death Differ 18:271–281
Stewart BW, Wild CP (2014) World cancer report 2014. World Health Organization, Geneva (Chapter 1.1, ISBN 92-832-0429-8)
Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y (2012) SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 31:4619–4629
Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A (2012) EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 20:3429–3456
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449
Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25:5603–5613
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939
Boyer B, Tucker GC, Valles AM, Gavrilovic J, Thiery JP (1989) Reversible transition towards a fibroblastic phenotype in a rat carcinoma cell line. Int J Cancer 4(suppl):69–75
Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S (2009) A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 137:87–98
Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, Li KC, Hong TM, Yang PC (2009) p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of slug. Nat Cell Biol 11:694–704
Shi Lei, Jackstadt Rene, Siemens Helge, Li Huihui, Kirchner Thomas, Hermeking Heiko (2014) p53-Induced miR-15a/16-1 and AP4 Form a double-negative feedback loop to regulate epithelial–mesenchymal transition and metastasis in colorectal cancer. Cancer Res 74:532–542
Yi J, Gong WD, Wang L, Ling R, Chen JH, Yun J (2005) VP22 fusion protein-based dominant negative mutant can inhibit hepatitis B virus replication. World J Gastroenterol 11:6429–6432
Liu XH, Zhang B, Guo Y, Wu L, Wu CY, Liang Q, Ye L, Tao KX, Wang GB, Chen JY (2012) The overexpression of AP4 as a prognostic indicator for gastric carcinoma. Med Oncol 29:871–877
Acknowledgments
This work was supported by the Seed Grant of City University of Hong Kong (Project no. 7004028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Chiu, SK. AP4 activates cell migration and EMT mediated by p53 in MDA-MB-231 breast carcinoma cells. Mol Cell Biochem 407, 57–68 (2015). https://doi.org/10.1007/s11010-015-2454-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2454-7