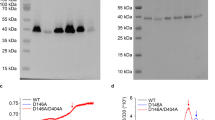
Abstract
In this work, we report the phenotypic and biochemical effects of deleting the C-terminal cytoplasmic portion of the NhaP2 cation/proton antiporter from Vibrio cholerae. While the deletion changed neither the expression nor targeting of the Vc-NhaP2 in an antiporter-less Escherichia coli strain, it resulted in a changed sensitivity of the host to sodium ions at neutral pH, indicating an altered Na+ transport through the truncated variant. When assayed in inside-out sub-bacterial vesicles, the truncation was found to result in greatly reduced K+/H+ and Na+/H+ antiport activity at all pH values tested and a greater than fivefold decrease in the affinity for K+ (measured as the apparent K m) at pH 7.5. Being expressed in trans in a strain of V. cholerae bearing a chromosomal nhaP2 deletion, the truncated nhaP2 gene was able to complement its inability to grow in potassium-rich medium at pH 6.0. Thus the residual K+/H+ antiport activity associated with the truncated Vc-NhaP2 was still sufficient to protect cells from an over-accumulation of K+ ions in the cytoplasm. The presented data suggest that while the cytoplasmic portion of Vc-NhaP2 is not involved in ion translocation directly, it is necessary for optimal activity and substrate binding of the Vc-NhaP2 antiporter.





Similar content being viewed by others
References
Resch CT, Winogrodzki JL, Patterson CT, Lind EJ, Quinn MJ, Dibrov P, Häse CC (2010) Putative Na+/H+ antiporter of Vibrio cholerae, Vc-NhaP2, mediates the specific K+/H+ exchange in vivo. Biochemistry 49:2520–2528
Quinn MJ, Resch CT, Sun J, Lind EJ, Dibrov P, Häse CC (2012) NhaP1 is a K+(Na+)/H+ antiporter required for growth of Vibrio cholerae at low extracellular pH. Microbiology (UK) 158:1094–1105
Resch CT, Winogrodzki JL, Häse CC, Dibrov P (2011) Insights into the biochemistry of the ubiquitous NhaP family of cation/H+ antiporters. Biochem Cell Biol 89:130–137
Saier MH Jr, Eng BH, Fard S, Garg J, Haggerty DA, Hutchinson WJ, Jack DL, Lai EC, Liu HJ, Nusinew DP, Omar AM, Pao SS, Paulsen IT, Quan JA, Sliwinski M, Tseng T, Wachi S, Young GB (1999) Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim Biophys Acta 1422:1–56
Orlowski J, Grinstein S (1997) Na+/H+ exchangers of mammalian cells. J Biol Chem 272:22373–22376
Shi H, Ishitani M, Kim C, Zhu J (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Hamada A, Hibino T, Nakamura T, Takabe T (2001) Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol 125:437–446
Waditee R, Hibino T, Tanaka Y, Nakamura T, Incharoensakdi A, Takabe T (2001) Halotolerant cyanobacterium Aphanothece halophytica contains an Na+/H+ antiporter, homologous to eukaryotic ones, with novel ion specificity affected by C-terminal tail. J Biol Chem 276:36931–36938
Waditee R, Buaboocha T, Kato M, Hibino T, Suzuki S, Nakamura T, Takabe T (2006) Carboxyl-terminal hydrophilic tail of a NhaP type Na+/H+ antiporter from cyanobacteria is involved in the apparent affinity for Na+ and pH sensitivity. Arch Biochem Biophys 450:113–121
Jones DT, Taylor WR, Thornton JM (1994) A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 33:3038–3049
Jones DT (2007) Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23:538–544
Nugent T, Jones DT (2009) Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics 10:159
Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT (2005) Protein structure prediction servers at University College London. Nucleic Acids Res 33:W36–W38
Buchan DWA, Ward SM, Lobley AE, Nugent TCO, Bryson K, Jones DT (2010) Protein annotation and modeling servers at University College London. Nucleic Acids Res 38:W563–W568
Ohyama T, Igarashi K, Kobayashi H (1994) Physiological role of chaA gene in sodium and calcium circulations at high pH in Escherichia coli. J Bacteriol 176:4311–4315
Mekalanos JJ, Swartz DJ, Pearson GD, Harford N, Groyne F, de Wilde M (1983) Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557
Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S (1989) Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ system(s). J Biol Chem 264:20297–202302
Hamashima H, Iwasaki M, Arai T (1995) A simple and rapid method for transformation of Vibrio species by electroporation. Methods Mol Biol 47:155–160
Dzioba-Winogrodzki J, Winogrodzki O, Krulwich TA, Boin MA, Häse CC, Dibrov P (2009) Characterization of the Vibrio cholerae multifunctional mrp-encoded cation/proton antiporter conferring resistance to bile salts in a heterologous host. J Mol Microbiol Biotechnol 16:176–186
Galili L, Rothman A, Kozachkov L, Rimon A, Padan E (2002) Transmembrane domain IV is involved in ion transport activity and pH regulation of the NhaA Na+/H+ antiporter of Escherichia coli. Biochemistry 41:609–617
Padan E, Tzubery T, Herz K, Kozachkov L, Rimon A, Galili L (2004) NhaA of Escherichia coli, as a model of a pH-regulated Na+/H+ antiporter. Biochim Biophys Acta 1658:2–13
Habibian R, Dzioba J, Barrett J, Galperin MY, Loewen PC, Dibrov P (2005) Functional analysis of conserved polar residues in Vc-NhaD, Na+/H+ antiporter of Vibrio cholerae. J Biol Chem 280:39637–39649
Taglicht D, Padan E, Schuldiner S (1991) Overproduction and Purification of a Functional Na+/H+ Antiporter Coded by nhaA (ant) from Escherichia coli. J Biol Chem 266:11289–11294
Dibrov P, Smith JJ, Young PG, Fliegel L (1997) Identification and characterization of the sod2 gene product in fission yeast. FEBS Lett 405:119–124
Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H (2005) Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435:1197–1202
Arkin IT, Xu H, Jensen MØ, Arbely E, Bennett ER, Bowers KJ, Chow E, Dror RO, Eastwood MP, Flitman-Tene R, Gregersen BA, Klepeis JL, Kolossváry I, Shan Y, Shaw DE (2007) Mechanism of Na+/H+ antiporting. Science 317:799–803
Padan E (2008) The enlightening encounter between structure and function in the NhaA Na+–H+ antiporter. Trends Biochem Sci 33:435–443
Lee BL, Sykes BD, Fliegel L (2013) Structural and functional insights into the cardiac Na+/H+ exchanger. J Mol Cell Cardiol 61:60–67
Häse C, Fedorova N, Galperin M, Dibrov P (2001) Sodium cycle in bacterial pathogens. Evidence from cross-genome comparisons. Microbiol Mol Biol Rev 65:353–370
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Acknowledgments
This research was supported by Grants from the Natural Sciences and Engineering Research Council of Canada (to JS, PD, JLW, and CTR). J. Stetefeld is a Canada Research Council Chair in Structural biology.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Contribution
EJW, CTR and GLO performed most of the experiments, contributed to data organization and manuscript review; JLW performed part of the gene construction, as well as growth experiments, reviewed the manuscript; JS designed some experiments, critically reviewed data, contributed to the writing of manuscript; PD designed most of the experiments, critically edited data, wrote and reviewed manuscript.
Rights and permissions
About this article
Cite this article
Wiens, E.J., Winogrodzki, J.L., Resch, C.T. et al. The C-terminal cytoplasmic portion of the NhaP2 cation–proton antiporter from Vibrio cholerae affects its activity and substrate affinity. Mol Cell Biochem 389, 51–58 (2014). https://doi.org/10.1007/s11010-013-1926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1926-x