Abstract
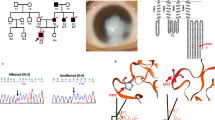
To detect the underlying genetic defects in two autosomal dominant congenital cataract (ADCC) families, having respectively twenty and four members affected with bilateral congenital cataract. Detailed family history and clinical data were recorded. Mutation screening in twenty three candidate genes including crystallins (CRYAA, CRYAB, CRYBA1/A3, CRYBA2, CRYBA4, CRYBB1, CRYBB2, CRYBB3, CRYGA, CRYGB, CRYGC, CRYGD, and CRYGS), gap junctional channels; connexins (GJA8, GJA3), beaded filament chain proteins (BFSP1, BFSP2), major intrinsic protein (MIP), lens intrinsic membrane protein-2 (LIM2), transcriptional factor (MAF), and in genes encoding for membrane-associated proteins (TMEM114, CHMP4B, EPHA2) was performed by bi-directional sequence analysis of the amplified products. In family A twenty members in six generations were affected by bilateral aculeiform type cataract and in family B four affected members in three generations had granular nuclear cataract. Mutation screening in already known candidate genes by sequence analyses revealed proline to threonine substitution at codon 23 (p.Pro23Thr) in CRYGD for aculeiform type cataract in family A. The family B with four members affected by granular nuclear cataract, however, could not be linked with any of these analyzed 23 candidate genes. The present study describes identification of p.Pro23Thr mutation in CRYGD for aculeiform type cataract in an ADCC family of Indian origin. The identical mutation has previously been reported to be linked with different phenotypes; lamellar cataract, cerulean cataract, coralliform cataract, flaky silica-like nuclear cataract and fasciculiform type cataract in different ADCC families. Interestingly, a mutation of different codon, i.e., p.Arg58His in CRYGD has been reported to be linked with aculeiform cataract in four different families; two from Switzerland, one from Macedonia and in a Mexican family. The findings in present study thus expand the genetic heterogeneity for aculeiform type cataract. Further, exclusion of these twenty three known candidate genes in family B having ADCC of granular nuclear type indicates the role of some other gene apart from for crystallins, gap junction channels, beaded filaments and membrane-associated proteins, and MAF for this phenotype.


Similar content being viewed by others
References
Lambert SR, Drack AV (1996) Infantile cataracts. Surv Ophthalmol 40:427–458
Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT (2004) Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol 49:300–315
Vanita V, Singh JR, Singh D (1999) Genetic and segregation analysis of congenital cataract in the Indian population. Clin Genet 56:389–393
Tardieu A (1988) Eye lens proteins and transparency: from light transmission theory to solution X-ray structural analysis. Annu Rev Biophys Biophys Chem 17:47–70
Wistow GJ, Piatigorsky J (1988) Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem 57:479–504
Jaffe NS, Horwitz J (1992) Lens and cataract. In: Podos SM, Yanoff M (eds) Text book of ophthalmology, vol 3. Gower Medical Publishing, New York, pp 56–57
Russell P, Meakin SO, Hohman T, Tsui LC, Breitman ML (1987) Relationship between proteins encoded by three human gamma-crystallin genes and distinct polypeptides in the eye lens. Mol Cell Biol 7:3320–3323
Vanita V, Singh D, Robinson PN, Sperling K, Singh JR (2006) A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am J Med Genet A 140:558–566
Santhiya ST, Shyam Manohar M, Rawlley D, Vijayalakshmi P, Namperumalsamy P, Gopinath PM, Loster J, Graw J (2002) Novel mutations in the gamma-crystallin genes cause autosomal dominant congenital cataracts. J Med Genet 39:352–358
Nandrot E, Slingsby C, Basak A, Cherif-Chefchaouni M, Benazzouz B, Hajaji Y, Boutayeb S, Gribouval O, Arbogast L, Berraho A, Abitbol M, Hilal L (2003) Gamma-D crystallin gene (CRYGD) mutation causes autosomal dominant congenital cerulean cataracts. J Med Genet 40:262–267
Mackay DS, Andley UP, Shiels A (2004) A missense mutation in the gammaD crystallin gene (CRYGD) associated with autosomal dominant “coral-like” cataract linked to chromosome 2q. Mol Vis 10:155–162
Burdon KP, Wirth MG, Mackey DA, Russell-Eggitt IM, Craig JE, Elder JE, Dickinson JL, Sale MM (2004) Investigation of crystallin genes in familial cataract, and report of two disease associated mutations. Br J Ophthalmol 88:79–83
Shentu X, Yao K, Xu W, Zheng S, Hu S, Gong X (2004) Special fasciculiform cataract caused by a mutation in the gammaD crystallin gene. Mol Vis 10:233–239
Zhang LY, Gong B, Tong JP, Fan DS, Chiang SW, Lou D, Lam DS, Yam GH, Pang CP (2009) A novel gammaD-crystallin mutation causes mild changes in protein properties but leads to congenital coralliform cataract. Mol Vis 15:1521–1529
Xu WZ, Zheng S, Xu SJ, Huang W, Yao K, Zhang SZ (2004) Autosomal dominant coralliform cataract related to a missense mutation of the gammaD-crystallin gene. Chin Med J (Engl) 117:727–732
Khan AO, Aldahmesh MA, Ghadhfan FE, Al-Mesfer S, Alkuraya FS (2009) Founder heterozygous P23T CRYGD mutation associated with cerulean (and coralliform) cataract in 2 Saudi families. Mol Vis 15:1407–1411
Yang G, Zhang G, Wu Q, Zhao J (2011) A novel mutation in the MIP gene is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis 17:1320–1323
Vogt A (1922) Weitere ergebnisse der spaltlampenmikroskopie des vorderen bulbsabschnittes. III. Angeborene und fru¨ therworbene Linsenvera¨nderungen. von Greafes Arch Ophthalmol 107:237–240
Parker C (1956) Spear cataract. Arch Ophthalmol 55:23–24
Farmer EJ, Swann PG (1983) Crystalline aculeiform cataract. J Am Optom Assoc 54:913–914
Héon E, Liu S, Billingsley G, Bernasconi O, Tsifildis C, Shorderet DF, Munier FL (1998) Gene localization for aculeiform cataract, on chromosome 2q33-35. Am J Hum Genet 63:921–926
Heon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, Lubsen N, Munier FL (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267
Zenteno JC, Morales ME, Moran-Barroso V, Sanchez-Navarro A (2005) CRYGD gene analysis in a family with autosomal dominant congenital cataract: evidence for molecular homogeneity and intrafamilial clinical heterogeneity in aculeiform cataract. Mol Vis 11:438–442
Plotnikova OV, Kondrashov FA, Vlasov PK, Grigorenko AP, Ginter EK, Rogaev EI (2007) Conversion and compensatory evolution of the gamma-crystallin genes and identification of a cataractogenic mutation that reverses the sequence of the human CRYGD gene to an ancestral state. Am J Hum Genet 81:32–43
Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King J, Benedek GB (2001) Crystal cataracts: human genetic cataract caused by protein crystallization. Proc Natl Acad Sci USA 98:6116–6120
Evans P, Wyatt K, Wistow GJ, Bateman OA, Wallace BA, Slingsby C (2004) The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin. J Mol Biol 343:435–444
Jung J, Byeon IJ, Wang Y, King J, Gronenborn AM (2009) The structure of the cataract-causing P23T mutant of human gammaD-crystallin exhibits distinctive local conformational and dynamic changes. Biochemistry 48:2597–2609
Pande A, Zhang J, Banerjee PR, Puttamadappa SS, Shekhtman A, Pande J (2009) NMR study of the cataract-linked P23T mutant of human gammaD-crystallin shows minor changes in hydrophobic patches that reflect its retrograde solubility. Biochem Biophys Res Commun 382:196–199
Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GCM (2002) Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet 11:33–42
Gill D, Klose R, Munier FL, McFadden M, Priston M, Billingsley G, Ducrey N, Schorderet DF, Héon E (2000) Genetic heterogeneity of the Coppock-like cataract: a mutation in CRYBB2 on chromosome 22q11.2. Invest Ophthalmol Vis Sci 41:159–165
Acknowledgments
We wish to thank the patients and their relatives for their cooperation. This study was in part supported by grant sanctioned from DBT, India BT/IN/German/13/VK/2010 and Bundesministerium für Bildung und Forschung BMBF, IND 10/036 under the framework of Indo-German bilateral cooperation for research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vanita, V., Singh, D. A missense mutation in CRYGD linked with autosomal dominant congenital cataract of aculeiform type. Mol Cell Biochem 368, 167–172 (2012). https://doi.org/10.1007/s11010-012-1355-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1355-2