Abstract
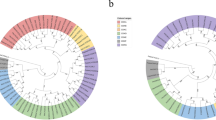
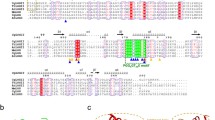
In all eukaryotes, the typical CK2 holoenzyme is an heterotetramer composed of two catalytic (CK2α and CK2α′) and two regulatory (CK2β) subunits. One of the distinctive traits of plant CK2 is that they present a greater number of genes encoding for CK2α/β subunits than animals or yeasts, for instance, in Arabidopsis and maize both CK2α/β subunits belong to multigenic families composed by up to four genes. Here, we conducted a genome-wide survey examining 34 different plant genomes in order to investigate if the multigenic property of CK2β genes is a common feature through the entire plant kingdom. Also, at the level of structure, the plant CK2β regulatory subunits present distinctive features as (i) they lack about 20 aminoacids in the C-terminal domain, (ii) they present a specific N-terminal extension of about 90 aminoacids that shares no homology with any previously characterized functional domain, and (iii) the acidic loop region is poorly conserved at the aminoacid level. Since there is no data about CK2β or holoenzyme structure in plants, in this study, we use human CK2β as a template to predict a structure for Zea mays CK2β1 by homology modeling and we discuss about possible structural changes in the acidic loop region that could affect the enzyme regulation.

Similar content being viewed by others
References
Lee Y, Lloyd AM, Roux SJ (1999) Antisense expression of the CK2 alpha-subunit gene in Arabidopsis. Effects on light-regulated gene expression and plant growth. Plant Physiol 119:989–1000
Sugano S, Andronis C, Ong MS, Green RM, Tobin EM (1999) The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc Natl Acad Sci USA 96:12362–12366
Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martínez MC (1999) Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J 19:655–666
Hidalgo P, Garreton V, Berrios CG, Ojeda H, Jordana X, Holuigue L (2001) A nuclear casein kinase 2 activity is involved in early events of transcriptional activation induced by salicylic acid in tobacco. Plant Physiol 125:396–405
Moreno-Romero J, Espunya M, Platara M, Ariño J, Martínez M (2008) A role for protein kinase CK2 in plant development: evidence obtained using a dominant-negative mutant. Plant J 55:118–130
Portolés S, Más P (2007) Altered oscillator function affects clock resonance and is responsible for the reduced day-length sensitivity of CKB4 overexpressing plants. Plant J 51:966–977
Riera M, Figueras M, López C, Goday A, Pagès M (2004) Protein kinase CK2 modulates developmental functions of the abscisic acid responsive protein Rab17 from maize. Proc Natl Acad Sci USA 101:9879–9884
Meggio F, Boldyreff B, Marin O, Marchiori F, Perich JW, Issinger O-G, Pinna LA (1992) The effect of polylysine on casein-kinase-2 activity is influenced by both the structure of the protein/peptide substrates and the subunit composition of the enzyme. Eur J Biochem 205:939–945
Bibby A, Litchfield DW (2005) The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2beta. Int J Biol Sci 1:67–79
Bolanos-Garcia VM, Fernandez-Recio J, Allende JE, Blundell TL (2006) Identifying interaction motifs in CK2[beta]—a ubiquitous kinase regulatory subunit. Trends Biochem Sci 31:654–661
Allende J, Allende C (1995) Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J 9:313–323
Lozeman F, Litchfield D, Piening C, Takio K, Walsh K, Krebs E (1990) Isolation and characterization of human cDNA clones encoding the alpha and the alpha′ subunits of casein kinase II. Biochemistry 29:8436–8447
Dobrowolska G, Boldyreff B, Issinger O-G (1991) Cloning and sequencing of the casein kinase 2 [alpha] subunit from Zea mays. Biochim Biophys Acta 1129:139–140
Peracchia G, Jensen AB, Culiáñez-Macià FA, Grosset J, Goday A, Issinger O-G, Pagès M (1999) Characterization, subcellular localization and nuclear targeting of casein kinase 2 from Zea mays. Plant Mol Biol 40:199–211
Riera M, Peracchia G, De Nadal E, Ariño J, Pagès M (2001) Maize protein kinase CK2: regulation and functionality of three β regulatory subunits. Plant J 25:365–374
Collinge MA, Walker JC (1994) Isolation of an Arabidopsis thaliana casein kinase II β subunit by complementation in Saccharomyces cerevisiae. Plant Mol Biol 25:649–658
Perales M, Portolés S, Más P (2006) The proteasome-dependent degradation of CKB4 is regulated by the Arabidopsis biological clock. Plant J 46:849–860
Mizoguchi T, Yamaguchi-Shinozaki K, Hayashida N, Kamada H, Shinozaki K (1993) Cloning and characterization of two cDNAs encoding casein kinase II catalytic subunits in Arabidopsis thaliana. Plant Mol Biol 21:279–289
Riera M, Peracchia G, Pagès M (2001) Distinctive features of plant protein kinase CK2. Mol Cell Biochem 227:119–127
Dennis MD, Browning KS (2009) Differential phosphorylation of plant translation initiation factors by Arabidopsis thaliana CK2 holoenzymes. J Biol Chem 284:20602–20614
Espunya MC, Lopez-Giraldez T, Hernan I, Carballo M, Martinez MC (2005) Differential expression of genes encoding protein kinase CK2 subunits in the plant cell cycle. J Exp Bot 56:3183–3192
Niefind K, Guerra B, Pinna LA, Issinger O-G, Schomburg D (1998) Crystal structure of the catalytic subunit of protein kinase CK2 from Zea mays at 2.1 Å resolution. EMBO J 17:2451–2462
Niefind K, Guerra B, Ermakowa I, Issinger O-G (2001) Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J 20:5320–5331
Chantalat L, Leroy D, Filhol O, Nueda A, Benitez MJ, Chambaz EM, Cochet C, Dideberg O (1999) Crystal structure of the human protein kinase CK2 regulatory subunit reveals its zinc finger-mediated dimerization. EMBO J 18:2930–2940
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Combet C, Jambon M, Deleage G, Geourjon C (2002) Geno3D: automatic comparative molecular modelling of protein. Bioinformatics 18:213–214
Rensing S, Lang D, Zimmer AD, Terry A, Salamov A et al (2008) The physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319:64–69
Flagel LE, Wendel JF (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183:557–564
Blanc G, Wolfe KH (2004) Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 16:1667–1678
Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422:433–438
Lespinet O, Wolf YI, Koonin EV, Aravind L (2002) The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome Res 12:1048–1059
Moore RC, Purugganan MD (2005) The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol 8:122–128
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH (2009) The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA 106:13875–13879
Marti-Renom MA, Stuart AC, Fiser As, Sanchez R, Melo F, Sali A (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct 29:291–325
Meggio F, Boldyreff B, Issinger O-G, Pinna LA (1994) Casein kinase 2 down-regulation and activation by polybasic peptides are mediated by acidic residues in the 55–64 region of the.beta-subunit. A study with calmodulin as phosphorylatable substrate. Biochemistry 33:4336–4342
Leroy D, Heriché J-K, Filhol O, Chambaz EM, Cochet C (1997) Binding of polyamines to an autonomous domain of the regulatory subunit of protein kinase CK2 induces a conformational change in the holoenzyme. J Biol Chem 272:20820–20827
Riera M, Pages M, Issinger O-G, Guerra B (2003) Purification and characterization of recombinant protein kinase CK2 from Zea mays expressed in Escherichia coli. Protein Expr Purif 29:24–32
Acknowledgments
M.R. was financed by I3P-CSIC2006 and CONSOLIDER (CSD2007-00057) from MICINN (Spain), I.C. V–B. by predoctoral fellowship FPI2007 from MICINN (Spain) and L. C–P. by Juan de la Cierva Programme, MICINN (Spain). This work was supported by grant BIO2009-13044-CO2-01 from MICINN (Spain).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velez-Bermudez, I.C., Irar, S., Carretero-Paulet, L. et al. Specific characteristics of CK2β regulatory subunits in plants. Mol Cell Biochem 356, 255–260 (2011). https://doi.org/10.1007/s11010-011-0971-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0971-6