Abstract
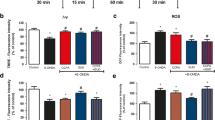
Dopamine is a neurotransmitter that has been related to mitochondrial dysfunction. In this study, striatal intact mitochondria and submitochondrial membranes were incubated with different dopamine concentrations, and changes on mitochondrial function, hydrogen peroxide, and nitric oxide production were evaluated. A 35% decrease in state 3 oxygen uptake (active respiration state) was found after 1 mM dopamine incubation. In addition, mitochondrial respiratory control significantly decreased, indicating mitochondrial dysfunction. High dopamine concentrations induced mitochondrial depolarization. Also, evaluation of hydrogen peroxide production by intact striatal mitochondria showed a significant increase after 0.5 and 1 mM dopamine incubation. Incubation with 0.5 and 1 mM dopamine increased nitric oxide production in submitochondrial membranes by 28 and 49%, respectively, as compared with control values. This study provides evidence that high dopamine concentrations induce striatal mitochondrial dysfunction through a decrease in mitochondrial respiratory control and loss of membrane potential, probably mediated by free radical production.



Similar content being viewed by others
References
Cohen G, Kesler N (1999) Monoamine oxidase inhibits mitochondrial respiration. Ann N Y Acad Sci 893:273–278
Cooper CE (2002) Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci 27(1):33–39
Murphy A (2000) Mitochondria in human disease. The Biochemist 22:19–24
Schinder AF, Olson EC, Spitzer NC, Montal M (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 16(19):6125–6133
Uchino H, Elmer E, Uchino K, Lindvall O, Siesjo BK (1995) Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol Scand 155(4):469–471
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73(3):1127–1137
Lai CT, Yu PH (1997) Dopamine- and L-beta-3,4-dihydroxyphenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol 53(3):363–372
Ben-Shachar D, Zuk R, Gazawi H, Ljubuncic P (2004) Dopamine toxicity involves mitochondrial complex I inhibition: implications to dopamine-related neuropsychiatric disorders. Biochem Pharmacol 67(10):1965–1974
Gluck M, Ehrhart J, Jayatilleke E, Zeevalk GD (2002) Inhibition of brain mitochondrial respiration by dopamine: involvement of H(2)O(2) and hydroxyl radicals but not glutathione-protein-mixed disulfides. J Neurochem 82(1):66–74
Gluck MR, Zeevalk GD (2004) Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson’s disease and catecholamine-associated diseases. J Neurochem 91(4):788–795
Khan FH, Sen T, Maiti AK, Jana S, Chatterjee U, Chakrabarti S (2005) Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implications for Parkinson’s disease. Biochim Biophys Acta 1741(1–2):65–74
Brown GC (2001) Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504(1):46–57
Czerniczyniec A, Bustamante J, Lores-Arnaiz S (2007) Dopamine enhances mtNOS activity: implications in mitochondrial function. Biochim Biophys Acta 1767(9):1118–1125
Lores-Arnaiz S, Coronel M, Boveris A (1999) Nitric oxide, superoxide and hydrogen peroxide production in brain mitochondria after haloperidol treatment. Nitric oxide 3:235–243
Boveris A, Arnaiz SL, Bustamante J, Alvarez S, Valdez L, Boveris AD, Navarro A (2002) Pharmacological regulation of mitochondrial nitric oxide synthase. Methods Enzymol 359:328–339
Boveris A, Costa LE, Cadenas E, Poderoso JJ (1999) Regulation of mitochondrial respiration by adenosine diphosphate, oxygen, and nitric oxide. Methods Enzymol 301:188–198
Estabrook R (1967) Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol 10:41–47
Bustamante J, Di Libero E, Fernandez-Cobo M, Monti N, Cadenas E, Boveris A (2004) Kinetic analysis of thapsigargin-induced thymocyte apoptosis. Free Radic Biol Med 37(9):1490–1498
Boveris A (1984) Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol 105:429–435
Jana S, Maiti AK, Bagh MB, Banerjee K, Das A, Roy A, Chakrabarti S (2007) Dopamine but not 3, 4-dihydroxy phenylacetic acid (DOPAC) inhibits brain respiratory chain activity by autoxidation and mitochondria catalyzed oxidation to quinone products: implications in Parkinson’s disease. Brain Res 1139:195–200
Boveris A (1998) Regulation of mitochondrial respiration by ADP, O2 and NO. Medicina (B Aires) 58(5 Pt 2):559–560
Zetterstrom T, Sharp T, Marsden CA, Ungerstedt U (1983) In vivo measurement of dopamine and its metabolites by intracerebral dialysis: changes after d-amphetamine. J Neurochem 41(6):1769–1773
Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK (2005) Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci 25(20):5013–5023
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56(3):331–349
Barzilai A, Melamed E, Shirvan A (2001) Is there a rationale for neuroprotection against dopamine toxicity in Parkinson’s disease? Cell Mol Neurobiol 21(3):215–235
Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, Langstrom B (1999) Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry 46(5):681–688
Przedborski S, Jackson-Lewis V, Muthane U, Jiang H, Ferreira M, Naini AB, Fahn S (1993) Chronic levodopa administration alters cerebral mitochondrial respiratory chain activity. Ann Neurol 34(5):715–723
Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW (1994) Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem 62(6):2484–2487
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59(3):527–605
Dawson VL, Dawson TM (1996) Nitric oxide neurotoxicity. J Chem Neuroanat 10(3–4):179–190
Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR (2006) Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology 31(3):493–505
Acknowledgments
This research was supported by grants from Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czerniczyniec, A., Bustamante, J. & Lores-Arnaiz, S. Dopamine modifies oxygen consumption and mitochondrial membrane potential in striatal mitochondria. Mol Cell Biochem 341, 251–257 (2010). https://doi.org/10.1007/s11010-010-0456-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0456-z