Abstract
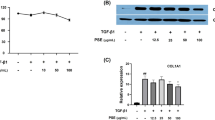
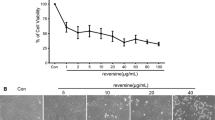
Hepatic stellate cells (HSCs) play an important role in the development of hepatic fibrosis. Heat shock protein 90 (Hsp90) is essential for the maturation and activity of a varied group of proteins involved in signal transduction and cell cycle regulation. In this study, we found that two Hsp90 inhibitors, VER-49009 and its analog VER-49009M, inhibited the proliferation of hepatic stellate cell line CFSC cells, and both of them induced G2 phase arrest in CFSC cells. Akt expression was decreased by the treatment of Hsp90 inhibitors in CFSC cells. Based on these findings, we propose that the inhibition of Hsp90 might be a rational approach in the prevention of liver fibrosis.




Similar content being viewed by others
References
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218
Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A (2005) Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 45:605–628. doi:10.1146/annurev.pharmtox.45.120403.095906
Friedman SL (2003) Liver fibrosis: from bench to bedside. J Hepatol 38:S38–S53. doi:10.1016/S0168-8278(02)00429-4
Neckers L (2002) Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 8:S55–S61. doi:10.1016/S1471-4914(02)02316-X
Powers MV, Workman P (2006) Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer 131:S125–S135. doi:10.1677/erc.1.01324
Grammatikakis N, Vultur A, Ramana CV, Siganou A, Schweinfest CW, Watson DK, Raptis L (2002) The role of Hsp90N, a new member of the Hsp90 family, in signal transduction and neoplastic transformation. J Biol Chem 277:8312–8320. doi:10.1074/jbc.M109200200
Neckers L, Ivy SP (2003) Heat shock protein 90. Curr Opin Oncol 15:419–424. doi:10.1097/00001622-200311000-00003
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172. doi:10.1016/j.tibs.2006.01.006
Workman P (2004) Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol Med 10:47–51. doi:10.1016/j.molmed.2003.12.005
Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P (2005) Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res 11:7023–7032. doi:10.1158/1078-0432.CCR-05-0518
Dymock BW, Barril X, Brough PA et al (2005) Novel, potent small-molecule inhibitors of the molecular chaperone Hsp90 discovered through structure-based design. J Med Chem 48:4212–4215. doi:10.1021/jm050355z
Greenwel P, Rubin J, Schwartz M, Hertzberg EL, Rojkind M (1993) Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest 69:210–217
Kang JX, Liu J, Wang J, He C, Li FP (2005) The extract of huanglian, a medicinal herb, induces cell growth arrest and apoptosis by upregulation of interferon-beta and TNF-alpha in human breast cancer cells. Carcinogenesis 26:1934–1939. doi:10.1093/carcin/bgi154
Okazaki I, Watanabe T, Hozawa S, Niioka M, Arai M, Maruyama K (2001) Reversibility of hepatic fibrosis: from the first report of collagenase in the liver to the possibility of gene therapy for recovery. Keio J Med 50:58–65
Friedman SL (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669. doi:10.1053/j.gastro.2008.03.003
Sreedhar AS, Kalmár E, Csermely P, Shen YF (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15. doi:10.1016/S0014-5793(04)00229-7
Saile B, Eisenbach C, Dudas J, El-Armouche H, Ramadori G (2004) Interferon-gamma acts proapoptotic on hepatic stellate cells (HSC) and abrogates the antiapoptotic effect of interferon-alpha by an HSP70-dependant pathway. Eur J Cell Biol 83:469–476. doi:10.1078/0171-9335-00409
Reif S, Lang A, Lindquist JN et al (2004) The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem 278:8083–8090. doi:10.1074/jbc.M212927200
Brough PA, Aherne W, Barril X et al (2008) 4,5-Diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer. J Med Chem 51:196–218. doi:10.1021/jm701018h
Acknowledgements
This study was supported by Grant 2007AA02Z301 of the National High Technology Research and Development Program of China (863 Program) and by Grant 07G603L056 of the New Drug Basic Research Program of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, X., Zhang, XD., Cheng, G. et al. Inhibition of hepatic stellate cell proliferation by heat shock protein 90 inhibitors in vitro. Mol Cell Biochem 330, 181–185 (2009). https://doi.org/10.1007/s11010-009-0131-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0131-4