Abstract
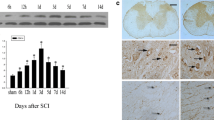
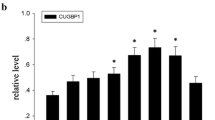
Protein kinases are critical signalling molecules for normal cell growth and development. CDK11p58 is a p34cdc2-related protein kinase, and plays an important role in normal cell cycle progression. However its distribution and function in the central nervous system (CNS) lesion remain unclear. In this study, we mainly investigated the protein expression and cellular localization of CDK11 during spinal cord injury (SCI). Western blot analysis revealed that CDK11p58 was not detected in normal spinal cord. It gradually increased, reached a peak at 3 day after SCI, and then decreased. The protein expression of CDK11p58 was further analyzed by immunohistochemistry. The variable immunostaining patterns of CDK11p58 were visualized at different periods of injury. Double immunofluorescence staining showed that CDK11 was co-expressed with NeuN, CNPase and GFAP. Co-localization of CDK11/active caspase-3 and CDK11/proliferating cell nuclear antigen (PCNA) were detected in some cells. Cyclin D3, which was associated with CDK11p58 and could enhance kinase activity, was detected in the normal and injured spinal cord. The cyclin D3 protein underwent a similar pattern with CDK11p58 during SCI. Double immunofluorescence staining indicated that CDK11 co-expressed with cyclin D3 in neurons and glial cells. Coimmunoprecipitation further showed that CDK11p58 and cyclin D3 interacted with each other in the damaged spinal cord. Thus, it is likely CDK11p58 and cyclin D3 could interact with each other after acute SCI. Another partner of CDK11p58 was β-1,4-galactosyltransferase 1 (β-1,4-GT 1). The co-localization of CDK11/β-1,4-GT 1 in the damaged spinal cord was revealed by immunofluorescence analysis. The cyclin D3-CDK4 complexes were also present by coimmunoprecipitation analysis. Taken together, these data suggested that both CDK11 and cyclin D3 may play important roles in spinal cord pathophysiology.








Similar content being viewed by others
References
Cernak I, Stoica B, Byrnes KR et al (2005) Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle 4:1286–1293
Di Giovanni S, Knoblach SM, Brandoli C et al (2003) Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol 53:454–468
Kato H, Takahashi A, Itoyama Y (2003) Cell cycle protein expression in proliferating microglia and astrocytes following transient global cerebral ischemia in the rat. Brain Res Bull 60:215–221
Padmanabhan J, Park DS, Greene LA et al (1999) Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J Neurosci 19:8747–8756
Strazza M, Luddi A, Brogi A et al (2004) Activation of cell cycle regulatory proteins in the apoptosis of terminally differentiated oligodendrocytes. Neurochem Res 29:923–931
Verdaguer E, Jimenez A, Canudas AM et al (2004) Inhibition of cell cycle pathway by flavopiridol promotes survival of cerebellar granule cells after an excitotoxic treatment. J Pharmacol Exp Ther 308:609–616
Wang F, Corbett D, Osuga H et al (2002) Inhibition of cyclin-dependent kinases improves CA1 neuronal survival and behavioral performance after global ischemia in the rat. J Cereb Blood Flow Metab 22:71–82
Bunnell BA, Heath LS, Adams DE et al (1990) Increased expression of a 58-kDa protein kinase leads to changes in the CHO cell cycle. Proc Natl Acad Sci USA 87:7467–7471
Ariza ME, Broome-Powell M, Lahti JM et al (1999) Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34(cdc2)-related PITSLRE protein kinases. J Biol Chem 274:28505–28513
Nelson MA, Ariza ME, Yang JM et al (1999) Abnormalities in the p34cdc2-related PITSLRE protein kinase gene complex (CDC2L) on chromosome band 1p36 in melanoma. Cancer Genet Cytogenet 108:91–99
Xiang J, Lahti JM, Grenet J et al (1994) Molecular cloning and expression of alternatively spliced PITSLRE protein kinase isoforms. J Biol Chem 269:15786–15794
Sauer K, Weigmann K, Sigrist S et al (1996) Novel members of the cdc2-related kinase family in Drosophila: cdk4/6, cdk5, PFTAIRE, and PITSLRE kinase. Mol Biol Cell 7:1759–1769
Li H, Grenet J, Valentine M et al (1995) Structure and expression of chicken protein kinase PITSLRE-encoding genes. Gene 153:237–242
Loyer P, Trembley JH, Lahti JM et al (1998) The RNP protein, RNPS1, associates with specific isoforms of the p34cdc2-related PITSLRE protein kinase in vivo. J Cell Sci 111:1495–1506
Berke JD, Sgambato V, Zhu PP et al (2001) Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron 32:277–287
Dickinson LA, Edgar AJ, Ehley J et al (2002) Cyclin L is an RS domain protein involved in pre-mRNA splicing. J Biol Chem 277:25465–25473
Cornelis S, Bruynooghe Y, Denecker G et al (2000) Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol Cell 5:597–605
Kidd VJ, Luo W, Xiang JL et al (1991) Regulated expression of a cell division control-related protein kinase during development. Cell Growth Differ 2:85–93
Niu Z, Shen A, Shen H et al (2005) Protein expression pattern of CDK11(p58) during testicular development in the mouse. Mol Cell Biochem 270:99–106
Zhang S, Cai M, Zhang S et al (2002) Interaction of p58 (PITSLRE), a G2/M-specific protein kinase, with cyclin D3. J Biol Chem 277:35314–35322
McEwen ML, Springer JE (2005) A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem 53:809–819
Zhang SW, Xu SL, Cai MM et al (2001) Effect of p58GTA on beta-1,4-galactosyltransferase 1 activity and cell-cycle in human hepatocarcinoma cells. Mol Cell Biochem 221:161–168
Bagui TK, Jackson RJ, Agrawal D et al (2000) Analysis of cyclin D3-cdk4 complexes in fibroblasts expressing and lacking p27(kip1) and p21(cip1). Mol Cell Biol 20:8748–8757
Kruman II, Wersto RP, Cardozo-Pelaez F et al (2004) Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41:549–561
Ino H, Chiba T (2001) Cyclin-dependent kinase 4 and cyclin D1 are required for excitotoxin-induced neuronal cell death in vivo. J Neurosci 21:6086–6094
Lahti JM, Xiang J, Heath LS et al (1995) PITSLRE protein kinase activity is associated with apoptosis. Mol Cell Biol 15:1–11
Shi J, Feng Y, Goulet AC et al (2003) The p34cdc2-related cyclin-dependent kinase 11 interacts with the p47 subunit of eukaryotic initiation factor 3 during apoptosis. J Biol Chem 278:5062–5071
Beyaert R, Kidd VJ, Cornelis S et al (1997) Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J Biol Chem 272:11694–11697
Yun X, Wu Y, Yao L et al (2007) CDK11(p58) protein kinase activity is associated with Bcl-2 down-regulation in pro-apoptosis pathway. Mol Cell Biochem 304:213–218
Petretti C, Savoian M, Montembault E et al (2006) The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep 7:418–424
Herzinger T, Reed SI (1998) Cyclin D3 is rate-limiting for the G1/S phase transition in fibroblasts. J Biol Chem 273:14958–14961
Boonen GJ, van Oirschot BA, van Diepen A et al (1999) Cyclin D3 regulates proliferation and apoptosis of leukemic T cell lines. J Biol Chem 274:34676–34682
Mendelsohn AR, Hamer JD, Wang ZB et al (2002) Cyclin D3 activates Caspase 2, connecting cell proliferation with cell death. Proc Natl Acad Sci USA 99:6871–6876
Li Z, Wang H, Zong H et al (2005) Downregulation of beta1,4-galactosyltransferase 1 inhibits CDK11(p58)-mediated apoptosis induced by cycloheximide. Biochem Biophys Res Commun 327:628–636
Mayeda A, Badolato J, Kobayashi R et al (1999) Purification and characterization of human RNPS1: a general activator of pre-mRNA splicing. EMBO J 18:4560–4570
Trembley JH, Hu D, Slaughter CA et al (2002) Casein kinase 2 interacts with cyclin-dependent kinase 11 (CDK11) in vivo and phosphorylates both the RNA polymerase II carboxyl-terminal domain and CDK11 in vitro. J Biol Chem 278:2265–2270
Trembley JH, Hu D, Hsu LC et al (2002) PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. J Biol Chem 277:2589–2596
Feng Y, Ariza ME, Goulet AC et al (2005) Death-signal-induced relocalization of cyclin-dependent kinase 11 to mitochondria. Biochem J 392:65–73
Li T, Inoue A, Lahti JM et al (2004) Failure to proliferate and mitotic arrest of CDK11(p110/p58)-null mutant mice at the blastocyst stage of embryonic cell development. Mol Cell Biol 24:3188–3197
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China Grant (No.30300099 and No.30770488), Natural Scientific Foundation of Jiangsu Province Grant (No.BK2003035 and No.BK2006547), College and University Natural Science Research Programme of Jiangsu province (No.03KJB180109 and No.04KJB320114) and Technology Guidance Plan for Social Development of Jiangsu Province Grant (BS2004526), Health Project of Jiangsu Province (H200632),”Liu Da Ren Cai Gao Feng” Financial Assistance of Jiangsu Province Grant (No.2).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Yuhong Ji and Feng Xiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ji, Y., Xiao, F., Sun, L. et al. Increased expression of CDK11p58 and cyclin D3 following spinal cord injury in rats. Mol Cell Biochem 309, 49–60 (2008). https://doi.org/10.1007/s11010-007-9642-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9642-z