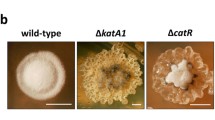
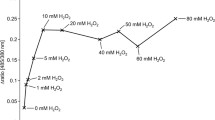
Abstract
Production and localization of endogenous hydrogen peroxide (H2O2) were investigated in strains of Xanthomonas by histochemical analysis under electron microscopy. Even though the levels of endogenous H2O2 production were different among various strains, the produced H2O2 was localized in the cell wall of all Xanthomonas strains tested. The impairment of the level of endogenous H2O2 accumulation resulted in a significantly decreased growth rate of bacteria, regardless if the difference of the H2O2 level is originally present between wild type strains or caused by mutation of the ahpC gene of Xanthomonas. The endogenous accumulation of H2O2 positively correlates with the cell division. Interestingly, the accumulated H2O2 was also localized in the mesosome-like structure and nucleoids during the cell division cycle. Furthermore, results revealed quantitative and dimensional changes of H2O2 accumulation in the two additional locations. These findings indicated that the additional locations of the accumulated H2O2 were closely associated with the process of cell division. Together, these results suggest that the endogenous H2O2 production plays an important role in cell proliferation of Xanthomonas.





Similar content being viewed by others
References
Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134:1784–1792
Hoidal JR (2001) Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol 25(6):661–663
Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116:81–88
Turpaev TK (2002) Reactive oxygen species and regulation of gene expression. Biochemistry (Moscow) 67(3):281–292
Burdon RH (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18(4):775–794
Stone JR, Collins T (2002) The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 9(4):231–238
González-Flecha B, Demple B (1995) Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem 270(23):13681–13687
Moy TI, Mylonakis E, Calderwood SB, Ausubel FM (2004) Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium. Infect Immun 72(8):4512–4520
Rolke Y, Liu SJ, Quidde T, Williamson B, Schouten A, Weltring KM, Siewers V, Tenberge KB, Tudzynski B, Tudzynski P (2004) Functional analysis of H2O2-generating systems in Botrytis cinerea: the major Cu–Zn-superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol Plant Pathol 5(1):17–27
Seaver LC, Imlay AJ (2004) Are respiratory enzymes the primary sources of intracellular hydrogen peroxide?. J Biol Chem 279(47):48742–48750
Huycke MM, Wendy J, Wack FM (1996) Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J Infect Dis 173:743–746
Thannickal JV, Fanburg LB (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:1005–1028
Li HY, Wang JS (1999) Release of active oxygen species from phytopathogenic bacteria and their regulation. Chinese Sci Bull 44(1):71–75
Leyns F, DeCleene M, Swings JG, Deley J (1984) The host range of the genus Xanthomonas. Bot Rev 50:308–356
Able AJ, Guest DI, Sutherland MW (2000) Hydrogen peroxide yields during the incompatible interaction of tobacco suspension cells inoculated with Phytophthora nicotianae. Plant Physiol 124: 899–910
Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9:209–221
Allen MM (1972) Mesosomes in blue-green algae. Arch Mikrobiol 84:199–206
Avakyan AA, Kats LN, Mineeva LA, Ratner EN, Gusev MV (1978) Electron microscopic data on mesosome-like and myelin-like structures in blue-green algae. Mikrobiologiya 47:739–744
Balkwill DL, Stevens SE Jr (1980) Effects of penicillin G on mesosome-like structures in Agmenellum quadruplicatum. Antimicrob Agents Chemother 17(3):506–509
Skorn M, Wirongrong W, Paiboon V, Suvit L, Mayuree F (2000) A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J Bacteriol 182(23):6845–6849
Storz G, Tartaglia LA, Ames BN (1990) Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248:189–194
Acknowledgments
We wish to thank Skorn Mongkolsuk for providing mutant strains for this work. Determination of hydrogen peroxide was accomplished with support from the Instrumental Analysis and Research Center, Lanzhou University. This work was supported by the National Natural Science Foundation of China (No. 30170238 and No. 30670070)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Li, H., Pang, X. et al. Localization changes of endogenous hydrogen peroxide during cell division cycle of Xanthomonas . Mol Cell Biochem 300, 207–213 (2007). https://doi.org/10.1007/s11010-006-9385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9385-2