Abstract
Objectives Vital to implementation of the World Health Organization (WHO) Safe Childbirth Checklist (SCC), designed to improve delivery of 28 essential birth practices (EBPs), is the availability of safe birth supplies: 22 EBPs on the SCC require one or more supplies. Mapping availability of these supplies can determine the scope of shortages and need for supply chain strengthening. Methods A cross-sectional survey on the availability of functional and/or unexpired supplies was assessed in 284 public-sector facilities in 38 districts in Uttar Pradesh, India. The twenty-three supplies were categorized into three non-mutually exclusive groups: maternal (8), newborn (9), and infection control (6). Proportions and mean number of supplies available were calculated; means were compared across facility types using t-tests and across districts using a one-way ANOVA. Log-linear regression was used to evaluate facility characteristics associated with supply availability. Results Across 284 sites, an average of 16.9 (73.5%) of 23 basic childbirth supplies were available: 63.4% of maternal supplies, 79.1% of newborn supplies, and 78.7% of infection control supplies. No facility had all 23 supplies available and only 8.5% had all four medicines assessed. Significant variability was observed by facility type and district. In the linear model, facility type and distance from district hospital were significant predictors of higher supply availability. Conclusions for Practice In Uttar Pradesh, more remote sites, and primary and community health centers, were at higher risk of supply shortages. Supply chain management must be improved for facility-based delivery and quality of care initiatives to reduce maternal and neonatal harm.
Similar content being viewed by others
Significance
The absence of a single essential supply may mean the difference between life and death for a mother or newborn. Lower level facilities and some districts are severely under supplied in Uttar Pradesh, which may be due to gaps in the supply chain or poor management at the facility or district level. This knowledge will support policy makers and government stakeholders in continuing to play an important role in the efforts to increase facility-based quality of care and improve maternal and newborn outcomes in Uttar Pradesh and globally.
Background
Despite global progress, maternal and neonatal mortality rates remain high. India has experienced improvements in both rates; however, Uttar Pradesh, India’s most populous state, has death rates well above the country averages. The maternal mortality ratio (MMR) is estimated at 201/100,000 (NITI Aayog 2018a) compared to 130/100,000 nationally; the neonatal mortality rate (NMR) is 40/1000 versus 31/1000 nationally (NITI Aayog 2018b).
Reduction in MMR and NMR is not an easy or straightforward goal: care and experiences in the prenatal, during childbirth, and postnatal periods need to be considered for significant and sustained change. To address health outcomes related to care during childbirth, a popular national strategy has been to encourage facility-based delivery assisted by a skilled provider (Hussein et al. 2004). In 2005, the Government of India implemented the conditional-cash transfer program called Janani Suraksha Yojana (JSY), promoting institutional delivery among women of lower socio-economic status. JSY provides direct cash payment to the mother as well the community health worker that supports care during and post-delivery (Janani Suraksha Yojana (JSY)|National Health Portal of India 2015). JSY has been associated with increased facility-based deliveries, vaccination rates, post-partum checkups, and breastfeeding around the time of delivery (Carvalho et al. 2014). However, the increase in facility-based delivery has not been associated with a significant reduction in MMR and NMR. This may be an indication of poor quality care. An assessment of the quality of care after JSY implementation in a nearby state noted chaotic, unsafe delivery rooms and lack of routine care, including poor techniques, disrespectful and sometimes harmful behavior of staff towards patients (Chaturvedi et al. 2015). Understanding the reasons behind poor quality of care is essential to addressing the problem.
Insufficient availability of supplies and equipment is a major barrier to healthcare workers’ ability to deliver quality care (Tsu 2004). These gaps in supplies and equipment are widely recognized as an important contributor to avoidable maternal and newborn deaths (Kerber et al. 2007; Wagstaff et al. 2004; Zupan 2005) and better equipped facilities were found to provide better quality care (Kruk et al. 2017). The United Nations Commission on Life Saving Commodities for Women and Children estimated that globally, over a 5-year period, 70,000 mothers could be saved by increasing access and delivery of oxytocin and magnesium sulfate. For newborns, increased access and delivery of antibiotics could save 1.2 million babies and correct use of functional resuscitation devices could save 336,000 babies (UN Commission on Life-Saving Commodities for Women and Children 2012). The Government of India (GoI) has made a commitment to make essential medicines available for free to patients (Foy 2012); its Maternal and Newborn Health Toolkit includes the necessary equipment for safe childbirth. However, a survey in 2014 of availability of medicines in Uttar Pradesh’s neighbor state Rajasthan found that availability varies depending on the health setting from 70% at the primary and community health center level to 88% at the district hospital level (Selvaraj et al. 2014b).
In Uttar Pradesh, supplies are allocated through the Central Medical Supply Department (CMSD), a division of the Medical Health Director General who reports to the Principal Secretary for Health and Family Welfare in the State. The CMSD manages the essential drug list and annually negotiates rate contracts with suppliers of consumables and medicines and quantity contracts for items for which the volume is more predictable, such as equipment. In the past, State funds have been used to procure 20% of the medicines from quantity contracts. For the other 80%, districts spend their state-allocated funds through the rate contracts. The Chief Medical Officer (CMO) of the District is in charge of maintaining the stock of supplies for the public health facilities in his or her district. The CMO “pulls” on the state procurement system by requesting supplies from the contracted supplier (quantity contracts) or by using their budget to purchase supplies directly through state-negotiated rate contracts. Allocation of supplies to the facilities in the CMO’s district is based on the requests made by lower level facilities (Selvaraj et al. 2014a).
The World Health Organization (WHO) Safe Childbirth Checklist (SCC) is designed to address gaps in quality of facility-based care through improving the provision of 28 evidence-based, essential birth practices (EBPs) around the time of childbirth. The BetterBirth Program tested the impact of a SCC-based, peer-coaching intervention on maternal and neonatal outcomes in Uttar Pradesh, India (Semrau et al. 2017). During coaching sessions, nurse-coaches identified and worked with frontline staff to overcome barriers to behavior change and utilization of the SCC. One pervasive barrier was a lack of supplies (Hirschhorn et al. 2015; Maisonneuve et al. 2018). Of the 28 practices on the SCC, 22 require supplies, including essential drugs (e.g. oxytocin), consumables (e.g. soap), and equipment (e.g. autoclave, bag and mask). Thus, supply stockouts pose a significant barrier to delivery of high quality maternal and neonatal care. During the BetterBirth trial, supplies were observed as an obstacle to providing care in 10% of coaching sessions (Hirschhorn et al. 2018).
For the BetterBirth Program and other facility-based quality improvement projects, facility data on supplies availability are vital to identify barriers faced by facility staff and identify effective implementation of improvement interventions. Supply data at the facility and district level are also critical for district and state leadership to understand local and system factors associated with supply availability. These data could be used to design and implement interventions to address broader supply system gaps. As part of the site selection process for the BetterBirth trial in Uttar Pradesh, we collected supply availability data in a cross-sectional study (Semrau et al. 2016, 2017). Here, we present information on the pattern of essential birth supplies availability across 284 public health facilities in Uttar Pradesh and associated facility characteristics.
Methods
Study Setting
In coordination with the Government of Uttar Pradesh and India, 38 of the 74 districts in Uttar Pradesh were selected as potential districts for the BetterBirth trial (Semrau et al. 2017). Districts that had security concerns, health threats from ongoing epidemics, extremely poor road access, or were part of the pilot testing were eliminated. All facilities in the 38 districts (320 facilities) were reviewed for facility classification and delivery load; 284/320 (88.8%) facilities were selected for site visits based on confirmation of facility classification as public-sector primary health centers (PHCs), community health centers (CHCs), or CHC/first referral units (CHC/FRU) and a government reported annual delivery load greater than 1000 in 2012 (Fig. 1). All eligible facilities in eligible districts were surveyed; 2–17 facilities (median 7) were surveyed per district.
Indian Public Health Standards’ guidelines (2012) state that primary health centers (PHCs) are responsible for covering primary health needs of 20,000 to 30,000 people and a target minimum of three deliveries a month (Maternal and Newborn Health Toolkit 2013). As needed, PHC staff refer cases to the community health center level (Indian Public Health Standards: Guidelines for Primary Health Centres 2012). Community health centers (CHCs) cover a population of approximately 80,000 of 120,000 individuals and have 4 PHCs that refer patients to each of them. CHCs are expected to provide a minimum of 10 delivers per month and CHC/FRUs, 20–50 deliveries per month (Maternal and Newborn Health Toolkit 2013). As originally designed, CHCs were planned to provide both basic emergency obstetric and newborn care (EmONC) and cesarean delivery; yet, many CHCs do not function with that capacity (Indian Public Health Standards: Guidelines for Community Health Centres 2012). A CHC/FRU is a facility that has been designated to provide 24-h comprehensive EmONC services: cesarean delivery and blood transfusions (Rural Health Statistics 2014).
Data Collection
The survey was developed based on the Government of India’s Facility Based Newborn Care Operational Guide published by the Ministry of Health and Family Welfare (2011). The final survey captured information on facility demographics, human resources, maternal and child mortality, and current availability of staff and supplies. These data were planned to be used for matching of facilities in the matched pair, cluster randomized control trial design of the BetterBirth trial. The supplies list was based on the resources needed for the successful implementation of the WHO SCC (Table 1) and represented a subset of the GoI Maternal and Newborn Health Toolkit equipment for labor and delivery plus four drugs listed on the SCC (antibiotics, oxytocin, magnesium sulfate, and Vitamin K). Except Vitamin K, the other three drugs were on the Uttar Pradesh essential drug list (2015).
The site visits were performed between July and October 2013 by four teams of two data collectors, including one nurse. Data collectors worked one-on-one with a facility staff member, either facility leader, labor room staff, and/or pharmacist, to locate and inspect supplies. During the assessment, data collectors directly observed and documented supply availability on paper surveys at each facility. Consumables were marked as “available” or “not available”; equipment was marked as “functional”, “not functional”, or “unavailable”; drugs were marked “available” if available and unexpired, or “unavailable” if not present or present but expired (Table 1). If an item was reported as “available” by the facility staff but could not be shown to the data collection team for inspection (e.g. the item was locked up), items were considered “unavailable.” Data were transferred from the paper survey to an online database to collate data across all facilities.
Ethics and Administrative Approval
This study was approved as a part of the BetterBirth Program, which obtained approvals from the Harvard T.H. Chan School of Public Health Institutional Review Board, World Health Organization Ethics Review Board, Population Services International Research Ethics Board, Community Empowerment Lab Institutional Ethics Board, and Jawaharlal Nehru Medical College-Belgaum ethics review board. The activities were conducted in partnership with the Government of Uttar Pradesh. Permission from the heads of each facility was also requested at each visit; all facilities agreed to participate.
Data Analysis
To measure general readiness for provision of care of laboring women and their neonates, supplies were grouped into composite measures (Table 1). We grouped supplies with similar function together and only one of the grouped items had to be available to be counted in the composite measure. For example, availability of either knife, blade, or scalpel (collected as separate supplies) was marked as “Yes” for appropriate supply to cut the umbilical cord. Maternal, newborn, and infection control composite measures were categorized and based on the WHO SCC organization.
Supplies availability was calculated as the proportion of total supplies available at each facility. Districts with 6 or more facilities (26 of the 75 districts), were included in the district-level analysis and differences between facilities in each district were calculated using an ANOVA. Mean number of supplies available and differences in availability by facility-type were compared with t-tests.
To measure availability of essential and life-saving drugs, we assessed the percentage of facilities that had availability of all four drugs (antibiotics, oxytocin, magnesium sulfate, and Vitamin K). IV fluids were not included because, alone, IV fluids do not prevent any of the major causes of death during childbirth.
Regression Analysis
The a priori chosen predictors of supplies availability were: facility type, delivery volume, catchment area population, distance to district hospital, and staff size. Distance to district hospital was chosen as it may impact the ease of supply distribution. Larger catchment area and delivery volume indicate higher turnover and potential strain on the facility. The number of birth attendants could be related to the workload of each staff member and opportunity to track and request supplies when needed.
To ascertain which of these predictors were associated with supply availability, we fit a robust generalized linear model (Wedderburn 1974) with a log link and fixed effects, along with bootstrapped (Efron and Tibshirani 1993) standard errors. This robust approach provides unbiased estimates of the ratio of mean number of supplies available for different covariate values, regardless of the underlying distribution of the number of supplies. Data were analyzed using STATA version 13.1 (College Station, Texas, USA); p values < 0.05 were considered statistically significant.
Results
Facility Demographics
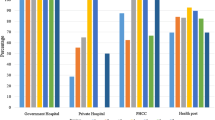
Of the 284 sites, including 62 CHC/FRUs, 137 CHCs, and 85 PHCs (Fig. 1), mean delivery load was 1998.8 births per year; mean distance to district hospital was 33.1 km; and mean catchment population was 257,100 persons (Table 2). Despite governmental targets, mean delivery load and staff levels did not vary by facility type. As expected, none of the 85 PHCs reported performing a cesarean delivery in the last year, while 3.65% of CHCs and 80% of CHC/FRUs reported at least one cesarean delivery in the last year (Table 2).
Individual Supplies
Of the 23 individual supplies assessed, only six were available in 90% or more of the facilities. The six most widely available supplies were any water supply at the facility for hand washing (running water or otherwise, 95.1%), gloves (95.1%), any blade to cut the cord (95.8%), a baby weigh scale (97.2%), chlorine bleach (90.5%), and antibiotics (90.5%). The following supplies were available in 50% or less of the facilities: functioning autoclave (46.8%), magnesium sulfate (17.6%), and vitamin K (18.3%) (Fig. 2). Of the remaining four essential medicines assessed in the survey, oxytocin was available at 63.4% of facilities and antibiotics were available at 90.5%. Only 24 (8.5%) of the facilities had all four medicines available.
Cumulative Supplies
None of the 284 facilities had all 23 essential supplies needed to safely deliver women and newborns. On average, facilities had 73.5% (mean number of 16.9, standard deviation (SD): ± 3.27) of supplies available. PHCs and CHCs had similar availability at 71.5% (mean 16.45, SD: ± 3.39) and 72% (mean 16.55, SD: ± 3.16) respectively, with CHC/FRUs having a significantly higher proportion of supplies (79.5%, mean 18.27, SD: ± 2.22, p < 0.05) (Table 3).
Maternal, Newborn, and Infection Control Supplies
Availability was also analyzed based on composite measures of supplies: maternal, newborn, and infection prevention (Table 3). Similar to the summary measure of all supplies, CHC/FRUs had significantly more supplies for maternal, newborn, and infection control compared to CHC and PHCs. Of the 8 maternal supplies, the average availability was 63.4% (PHCs 60.8%, CHCs: 62%, CHC/FRUs: 70%). Of the 11 newborn supplies, the average availability was 79.1% (PHCs: 75.2%, CHCs: 78.2%, CHC/FRUs: 84.3%). For infection prevention, the average availability was 78.7% (PHCs: 75.8%, CHCs: 76.2%, CHC/FRUs 88.2%.)
District-Level Availability
Because the supply chain depends in part on district-level systems, we assessed supply availability at the district level. Average facility supply availability ranged from 58 to 70% (Fig. 3). Significant differences were seen in average availability between districts (p < 0.05).
Regression Analysis
In fitting the robust generalized log-linear model, only facility type and delivery volume were significant predictors of supply availability. Specifically, facility type CHC/FRU was associated with an 11.1% higher mean supply availability (ratio of means = 1.11; p < 0.001) compared with CHC. Distance to district hospital was negatively associated with supply availability (ratio of means = 0.99, p = 0.04). As sites were further away from the district hospital, supplies were less available. Delivery volume, catchment area, and number of birth attendants were not correlated with supply availability (Table 4).
Discussion
Lack of availability of essential birth supplies was pervasive across the 284 facilities in Uttar Pradesh and varied by district. In the 284 facilities assessed, none of the facilities had all 23 supplies and only 8% of facilities had all four of the essential drugs assessed. Facility type, district, and distance to the nearest district hospital were significantly correlated with supply availability. These gaps highlight the need to include a systems-based approach to improving critical inputs, such as supplies and staff, in efforts to reduce maternal and neonatal harm.
Not surprisingly, higher-level facilities were more likely to have more supplies overall and across the composite measures. The improved availability of supplies in the CHC/FRUs may be due to focus given to the facilities as they were upgraded from a CHC to a CHC/FRU. In these data, PHCs had a far larger catchment area and higher number of deliveries than initially designed to manage. Here, we saw similar levels of supplies although we expected differences based on delivery load.
The district in which the facility is located was also significantly correlated with supply availability. The centralized system of the CMSD imposes many limitations on the nimbleness of the state and districts to respond to delivery load increases, epidemics, or quality improvement programs. The finding of district-level variability may reflect differences in leadership in that district, including their ability to work within the CMSD restrictions, manage funds, and develop alternate solutions. For example, in a given district, a Chief Medical Officer and his/her staff may have stronger skills and systems for managing supplies through seasonality of birth or unexpected surges in delivery volumes. Other factors influencing district-level supply availability may include proximity and the quality of roads, which is a significant challenge in many parts of India, including Uttar Pradesh (John 2014). Furthermore, closer proximity of a facility to the district hospital was associated with increased supply availability, potentially reflecting ease of getting supplies to the facility from the district hospital.
Across the 284 facilities, delivery volume was not correlated with supplies. This finding was in contrast to results from a study of 124 birth centers in 26 African and 15 Asian countries where low-volume facilities suffered the most severe shortages with only 13 of 23 surveyed supplies available (Spector et al. 2013). The surveyed facilities in this study were not particularly low volume, with averages of 1908 deliveries per year for PHCs and 2089 deliveries per year for CHCs, or an average of 5–six deliveries a day.
The poor availability of the four essential medicines assessed—oxytocin, magnesium sulfate, antibiotics, and Vitamin K—in only 8.5% (24/284) of facilities was extremely concerning, although some of the drugs (antibiotics and oxytocin) were more widely available. Use of oxytocin to augment labor may be an underlying cause of the limited stock (Iyengar et al. 2008; Sharan et al. 2005). A study in Northern Karnataka found similar levels of availability of oxytocin, but higher levels of availability of magnesium sulfate (Jayanna et al. 2016). Potentially, perverse incentives, such as requirement to pay for expired medications, may result in stock shortages. These findings are consistent with other studies from Northern India showing significant gaps in availability of medicines at public health facilities (Prinja et al. 2015). Furthermore, this work is consistent with studies that cite lack of supplies as one of the major reasons for not performing basic obstetric emergency care (Sabde et al. 2016) and that better availability of supplies is correlated with better care (Kruk et al. 2017).
Uttar Pradesh has a procurement system and funds to supply the necessary drugs for each patient. However, the implementation of this system faces challenges: the gaps we have observed, especially in drugs, were significant. Poor maternal and neonatal outcomes in Uttar Pradesh make the timely and appropriate delivery of essential supplies vital to improving quality of care for mothers and their newborns. The Government of Uttar Pradesh along with the World Bank are working to streamline the drug supplies and inventory in the state of UP and have specifically established the Uttar Pradesh Health System Strengthening Project (UPHSSP) to “improve the efficiency, quality, and accountability of health services delivery in Uttar Pradesh by strengthening the State Health Department’s management and systems capacity” (Uttar Pradesh Health System Strengthening Project 2013). Key for this group will be the ability to connect policy with supply availability at the point of care. For example, efforts during the BetterBirth trial to improve supply availability though coaching made modest improvements (Maisonneuve et al. 2018). Yet, more is required to create a procurement system that is reliable and sustainable. Governments must connect policy at the country and state levels and create a supportive and functional system to encourage frontline staff and managers to prevent stockouts.
Our study has a number of limitations. As a cross-sectional study, the data provided a snapshot of supply availability and readiness to utilize the WHO Safe Childbirth Checklist across Uttar Pradesh with supply availability defined as present on the day of the visit. This approach, therefore, did not provide longitudinal data on the frequency and severity of stockouts. Due to decisions to eliminate facilities with extremely poor road access, this study may have underestimated overall availability. Not all supplies needed for the SCC were assessed—including thermometer, stethoscope, fetoscope or Doppler, pads, and HIV test. Thus, we were not able to comment on gaps or availability of these supplies. The assessment tool did not allow for potential substitutes of oxytocin since the survey supply list was based on WHO Safe Childbirth Checklist requirements. For example, misoprostol would not be counted as a substitute for oxytocin; this decision was supported by a recent meta-analysis suggesting that misoprostol is not as effective as oxytocin for prevention of hemorrhage (Mousa et al. 2014).
Conclusion
Despite high availability of supplies in some facilities, significant room for improvement remains; the absence of a single essential supply may lead to poor outcomes for mothers and newborns. Lower level facilities and some districts are severely undersupplied in Uttar Pradesh, which may be due to gaps in the supply chain or poor management at the facility or district level. Ensuring that leaders at the facility, district and state levels have access to up to date supply information is a critical step to address the supply gaps needed to improve quality of care. UPHSSP has promise to ensure the greater availability of safe birth supplies in UP and should consider the role of facility and district leaders in this initiative and the need for them to be empowered to develop and implement action plans to improve supply availability. Further assessment of the functioning of the supply chain from the facility to district and state level, especially where variability occurs, is needed to identify areas of best practices and where policy or other interventions are needed. This knowledge can support policy makers and government stakeholders in continuing to focus efforts to increase facility-based quality of care and improve maternal and newborn outcomes in Uttar Pradesh and globally.
Abbreviations
- CHC/FRU:
-
Community Health Center/First Referral Unit
- CHC:
-
Community Health Center
- CMSD:
-
Central Medical Supply Department
- EBP:
-
Essential birth practices
- EmONC:
-
Emergency obstetric and newborn care
- GoI:
-
Government of India
- GoUP:
-
Government of Uttar Pradesh
- HIV:
-
Human immunodeficiency virus
- JSY:
-
Janani Suraksha Yojana
- PHC:
-
Primary Health Center
- SCC WHO:
-
Safe childbirth checklist
- UP:
-
Uttar Pradesh
- UPHSSP:
-
Uttar Pradesh Health System Strengthening Project
- WHO:
-
World Health Organization
References
Carvalho, N., Thacker, N., Gupta, S. S., & Salomon, J. A. (2014). More evidence on the impact of india’s conditional cash transfer program, Janani Suraksha Yojana: Quasi-experimental evaluation of the effects on childhood immunization and other reproductive and child health outcomes. PLoS ONE. https://doi.org/10.1371/journal.pone.0109311.
Chaturvedi, S., Costa, A. D., & Raven, J. (2015). Does the Janani Suraksha Yojana cash transfer programme to promote facility births in India ensure skilled birth attendance? A qualitative study of intrapartum care in Madhya Pradesh. Global Health Action. https://doi.org/10.3402/gha.v8.27427.
Efron, B., & Tibshirani, R. J. (1993). An introduction to the bootstrap. Boca Raton: Chapman & Hall/CRC.
Foy, H. (2012). India to give free generic drugs to hundreds of millions. Reuters. Retrieved from https://www.reuters.com/article/us-india-drugs-idUSBRE8630PW20120705. Retrieved 05 July 2012
Hirschhorn, L. R., Krasne, M., Maisonneuve, J., Kara, N., Kalita, T., Henrich, N., et al. (2018). Integration of the opportunity-ability-motivation behavior change framework into a coaching-based WHO Safe Childbirth Checklist program in India. International Journal Gynaecology Obstetrics 142 (3) 321–328. https://doi.org/10.1002/ijgo.12542.
Hirschhorn, L. R., Semrau, K., Kodkany, B., Churchill, R., Kapoor, A., Spector, J., et al. (2015). Learning before leaping: Integration of an adaptive study design process prior to initiation of BetterBirth, a large-scale randomized controlled trial in Uttar Pradesh, India. Implementation Science, 10, 117. https://doi.org/10.1186/s13012-015-0309-y.
Hussein, J., Bell, J., Nazzar, A., Abbey, M., Adjei, S., & Graham, W. (2004). The skilled attendance index: Proposal for a new measure of skilled attendance at delivery. Reproductive Health Matters, 12(24), 160–170. https://doi.org/10.1016/S0968-8080(04)24136-2.
Indian Public Health Standards: Guidelines for Community Health Centres. (2012). New Delhi: Ministry of Health & Family Welfare Government of India.
Indian Public Health Standards: Guidelines for Primary Health Centres. (2012). New Delhi: Ministry of Health & Family Welfare Government of India.
Iyengar, S. D., Iyengar, K., Martines, J. C., Dashora, K., & Deora, K. K. (2008). Childbirth practices in rural Rajasthan, India: Implications for neonatal health and survival. Journal of Perinatology, 28, S23–S30. https://doi.org/10.1038/jp.2008.174.
Janani Suraksha Yojana (JSY) | National Health Portal of India. (2015). Retrieved from https://www.nhp.gov.in/janani-suraksha-yojana-jsy-_pg.
Jayanna, K., Bradley, J., Mony, P., Cunningham, T., Washington, M., Bhat, S., et al. (2016). Effectiveness of onsite nurse mentoring in improving quality of institutional births in the primary health centres of high priority districts of Karnataka, South India: A cluster randomized trial. PLoS ONE. https://doi.org/10.1371/journal.pone.0161957.
John, S. (2014). Improving connectivity across rural India. Retrieved from http://www.worldbank.org/en/results/2014/04/10/improving-connectivity-roads-rural-india.
Kerber, K. J., de Graft-Johnson, J. E., Bhutta, Z. A., Okong, P., Starrs, A., & Lawn, J. E. (2007). Continuum of care for maternal, newborn, and child health: From slogan to service delivery—ScienceDirect. The Lancet, 370(9595), 1358–1369.
Kruk, M. E., Chukwuma, A., Mbaruku, G., & Leslie, H. H. (2017). Variation in quality of primary-care services in Kenya, Malawi, Namibia, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bulletin World Health Organization, 95, 408–418. https://doi.org/10.2471/BLT.16.175869.
Maisonneuve, J. J., Semaur, K. E. A., Maji, P., Pratap Singh, V., Miller, K. A., Solsky, I., et al. (2018). Effectiveness of a WHO safe childbirth checklist coaching-based intervention on the availability of essential birth supplies in Uttar Pradesh, India. International Journal of Quality in Health Care. https://doi.org/10.1093/intqhc/mzy086.
Maternal and Newborn Health Toolkit. (2013). New Delhi: Government of India.
Mousa, H. A., Blum, J., Senoun, A. E., Shakur, G. H., & Alfirevic, Z. (2014). Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD003249.pub3.
National Institution for Transforming India (NITI) Aayog. (2018a). Maternal Mortality Ratio (MMR) (per 100000 live births) (2018b). Government of India. Retrieved from http://niti.gov.in/content/maternal-mortality-ratio-mmr-100000-live-births.
National Institution for Transforming India (NITI) Aayog. (2018b). Neo Natal Mortality Rate (NMR) (per 1000 live births) Government of India. Retrieved from http://niti.gov.in/content/neo-natal-mortality-rate-nmr-1000-live-births.
Prinja, S., Bahuguna, P., Tripathy, J. P., & Kumar, R. (2015). Availability of medicines in public sector health facilities of two North Indian States. BMC Pharmacology and Toxicology, 16, 43. https://doi.org/10.1186/s40360-015-0043-8.
Rural Health Statistics. (2014). New Delhi: Government of India.
Sabde, Y., Diwan, V., Randive, B., Chaturvedi, S., Sidney, K., Salazar, M., & De Costa, A. (2016). The availability of emergency obstetric care in the context of the JSY cash transfer programme in Madhya Pradesh, India. BMC Pregnancy Childbirth, 16(1), 116. https://doi.org/10.1186/s12884-016-0896-x.
Selvaraj, S., Mukhopadyay, I., Bhat, P., Kumar, P., Aisola, M., Chokshi, M., et al. (2014a). 5.1 Description of different procurement models in India. In Universal Access to Medicines in India: A Baseline Evaluation of the Rajasthan Free Medicines Scheme (pp. 45–47). World Health Organization Country Office for India.
Selvaraj, S., Mukhopadyay, I., Bhat, P., Kumar, P., Aisola, M., Chokshi, M., et al. (2014b). 6.3.1 Availability and stock-out across districts. In Universal Access to Medicines in India: A Baseline Evaluation of the Rajasthan Free Medicines Scheme (pp. 70). World Health Organization Country Office for India.
Semrau, K. E. A., Hirschhorn, L. R., Kodkany, B., Spector, J. M., Tuller, D. E., King, G., et al. (2016). Effectiveness of the WHO Safe Childbirth Checklist program in reducing severe maternal, fetal, and newborn harm in Uttar Pradesh, India: Study protocol for a matched-pair, cluster-randomized controlled trial. Trials, 17, 576. https://doi.org/10.1186/s13063-016-1673-x.
Semrau, K. E. A., Hirschhorn, L. R., Singh, V. P., Saurastri, R., Sharma, N., Tuller, D. E., et al. (2017). Outcomes of a coaching-based WHO safe childbirth checklist program in India. New England Journal of Medicine, 14(24), 2313–2324.
Sharan, M., Strobino, D., & Ahmed, S. (2005). Intrapartum oxytocin use for labor acceleration in rural India. International Journal of Gynecology & Obstetrics, 90(3), 251–257. https://doi.org/10.1016/j.ijgo.2005.05.008.
Spector, J. M., Reisman, J., Lipsitz, S., Desai, P., & Gawande, A. A. (2013). Access to essential technologies for safe childbirth: A survey of health workers in Africa and Asia. BMC Pregnancy and Childbirth, 13, 43.
Tsu, V. D. (2004). New and underused technologies to reduce maternal mortality—ScienceDirect. The Lancet, 363(9402), 75–76.
UN Commission on Life-Saving Commodities for Women and Children. (2012). Commissioners’ Report September 2012. Retrieved from New York: https://www.unfpa.org/sites/default/files/pub-pdf/FinalUNCommission Report_14sept2012.pdf.
Uttar Pradesh Health System Strengthening Project. (2013). Retrieved from http://uphssp.org.in/.
Wagstaff, A., Bustreo, F., Bryce, J., Claeson, M., & Group, WHO-World Bank Child Health and Poverty Working Group (2004). Child health: Reaching the poor. American Journal of Public Health, 94(5), 726–736.
Wedderburn, R. W. M. (1974). Quasi-likelihood functions, generalized linear models, and the Gauss—Newton method. Biometrika, 61(3), 439–447.
Zupan, J. (2005). Perinatal mortality in developing countries. New England Journal of Medicine, 352, 2047–2048.
Acknowledgements
The authors would like to acknowledge the BetterBirth Team, especially the data collectors who traveled far and wide to collect this data. The authors would also like to thank the Government of India and Government of Uttar Pradesh for their support for the BetterBirth Program.
Funding
This work was generously funded through a grant from the Bill & Melinda Gates Foundation (Grant No. OPP1017378). The Bill & Melinda Gates Foundation reviewed the study design and sample size calculations for the BetterBirth Trial, but was not involved in data collection, management, analysis, interpretation of the data, writing the manuscript, or the decision to submit findings for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts except AAG receives royalties for books and essays, including on improving quality and delivery of health care using checklists.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Galvin, G., Hirschhorn, L.R., Shaikh, M. et al. Availability of Safe Childbirth Supplies in 284 Facilities in Uttar Pradesh, India. Matern Child Health J 23, 240–249 (2019). https://doi.org/10.1007/s10995-018-2642-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-018-2642-7