Abstract
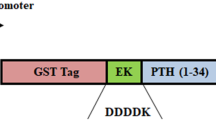
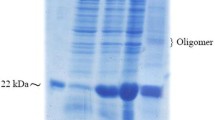
Several human diseases arise from abnormality in functional proteins production in different tissues. Recombinant protein drugs produced in special hosts are the most important solution for this problem. Efficient extraction and purification of recombinant protein from the host proteins is the fundamental stage in recombinant drug manufacture process. Teriparatide, a 34 amino acid peptide, is recombinant FDA approved drug used for osteoporosis stimulates the bone formation and rises its mass. In this research, 6his- E. coli K12β-gal (236-288)-hPTH (1-34) fusion protein was expressed by E. coli BL21 (DE3), extracted with combined chemical and sonication approach led to within 97.7 7% total proteins solubilization. Purification was done during two step chromatography and one enzymatic cleavage for rhPTH (1-34) separation. Approximately, 350 mg of fusion protein was obtained from 1 l culture volume after Ni2+ affinity. Enterokinase enzyme was used for fusion protein cleavage and recombinant teriparatide (1-34) was purified by cation exchange HiTrap SP XL chromatography column. Concentration of purified rhPTH (1-34) was estimated about 105 mg/1 l culture. Biological activity of rhPTH (1-34) was analyzed with serum calcium measurement in Vistar rat. The results were shown the rhPTH (1-34) was purified in this research has biological activity as equal as commercial drug. Efficient purification of active rhPTH (1-34) during the two step simple chromatography method is very precious in the industry of recombinant protein production.







Similar content being viewed by others
References
Bakhtiari N, Bayat ZA, Sagharidouz S, Vaez M (2017) Overexpression of recombinant human teriparatide, rhPTH(1-34) in Escherichia coli (E.coli): an innovative gene fusion approach. Avicenna J Med Biotechnol 9:19–22
Bornhorst JA, Falke JJ (2000) Purification of proteins using polyhistidine affinity tags. Methods Enzymol 326:245–254
Cheng ML, Gupta V (2012) Teriparatide—indications beyond osteoporosis. Indian J Endocrinol Metab 16:343–348
Ford CF, Suominen I, Glatz SE (1991) Fusion tails for the recovery and purification of recombinant proteins. Protein Expr Purif 2:95–107
Fu XY, Tong WY, Wei DZ (2005) Extracellular production of human parathyroid hormone as a thioredoxin fusion form in Escherichia coli by chemical permeabilization combined with heat treatment. Biotechnol Prog 21:429–435
Gangireddy SR, Madhavi RD, Ravikanth KR, Reddy PK, Konda VR, Rao KRS et al (2010) High yield expression of human recombinant PTH (1-34). Curr Trends Biotechnol Pharm 4:568–577
Gao X, Ma W, Dong H, Yong Z, Su R (2014) Establishing a rapid animal model of osteoporosis with ovariectomy plus low calcium diet inrats. Int J Clin Exp Pathol 7:5123–5128
Gardella TJ, Rubin D, Abou-Samra AB, Keutmann HT, Potts JT, Kronenberg HM et al (1990) Expression of human parathyroid hormone-(1-84) in Escherichia coli as a factor X-cleavable fusion protein. J Biol Chem 265:15854–15859
Hamedifar H, Salamat F, Saffarion M, Ghiasi M, Hosseini A, Lahiji H, Nouri Z, Arfae H, Mahboudi F (2013) Novel approach for high level expression of soluble recombinant human parathyroid hormone (rhPTH 1-34) in Escherichia coli. Avicenna J Med Biotechnol 5:193–201
Liu Q, Lin J, Liu M, Tao X, Wei D, Ma X, Yang S (2007) Large scale preparation of recombinant human parathyroid hormone 1-84 from Escherichia coli. Protein Expr Purif 54:212–219
Magdeldin S, Moser A (2012) Affinity chromatography: principles and applications, affinity chromatography. In: Magdeldin S (ed) Affinity chromatography. InTech, Rijeka, pp 1–28
Marcus R (2011) Present at the beginning: a personal reminiscence on the history of teriparatide. Osteoporos Int 22:2241–2248
Morelle G, Mayer H (1988) Increased synthesis of human parathyroid hormone in Escherichia coli through alterations of the 5′ untranslated region. Biochim Biophys Acta 950:459–462
Murby M, Cedergren L, Nilsson J, Nygren PA, Hammarberg B, Nilsson B, et al (1991) Stabilization of recombinant proteins from proteolytic degradation in Escherichia coli using a dual affinity fusion strategy. Biotechnol Appl Biochem 14:336–346
Murray TM, Rao LG, Divieti P, Bringhurst FR (2005) Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands. Endocr Rev 26:78–113
Oldenburg KR, D’Orfani AL, Selick HE (1994) A method for the high-level expression of a parathyroid hormone analog in Escherichia coli. Protein Expr Purif 5:278–284
Oshika Y, Yamada T, Nakagawa S, Fujishima A, Kawase M, Ishibashi Y et al (1994) Human parathyroid hormone: efficient synthesis in Escherchia coli using a synthetic gene, purification and characterization. Int J Pept Protein Res 43:441–447
Peternel Š (2013) Bacterial cell disruption: a crucial step in protein production. New Biotechnol 30:250–254
Sattayasai N (2012) Protein purification. In: Ekinci D (ed) Chemical biology. InTech, Rijeka, pp 3–18
Singh A, Upadhyay V, Panda A (2015) Solubilization and refolding of inclusion body proteins. Methods Mol Biol 1258:283–291
Smith ET, Johnson DA (2013) Human enteropeptidase light chain: bioengineering of recombinants and kinetic investigations of structure and function. Protein Sci 22:577–585
Vad R, Nafstad E, Dahl LA, Gabrielsen OS (2005) Engineering of a Pichia pastoris expression system for secretion of high amounts of intact human parathyroid hormone. J Biotechnol 116:251–260
Xiu Z, Li M, Zhou S, Dou H, Zhou H, Chen C (2002) A new method for the preparation of human parathyroid hormone 1-34 peptides. Biotechnol Appl Biochem 36:111–117
Xu XC, Zhong SD, Kai F, Li LR, Liu C, Liu B, Bao JK (2008) Preparation and characterization of a novel recombinant human parathyroid hormone (1-34) analog (Gly1-Gln26-rhPTH (1-34)) with enhanced biological activity. Protein Pept Lett 15:854–860
Acknowledgements
This project was carried out at the Laboratories of Iranian Research Organization for Science and Technology (IROST). We are grateful for the cooperation of this organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national and institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Abbaszadeh, S., Bakhtiari, N. & Amini-Bayat, Z. Simple and Effective Purification of Recombinant Peptide Drug, hPTH (1-34), Expressed in E. coli Host. Int J Pept Res Ther 25, 419–425 (2019). https://doi.org/10.1007/s10989-018-9685-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9685-x