Abstract
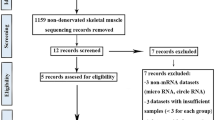
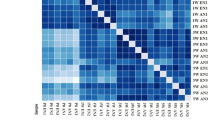
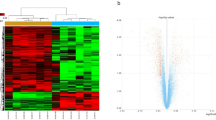
In obstetric brachial plexus palsy (OBPP), irreversible muscle atrophy occurs much faster in intrinsic muscles of the hand than in the biceps. To elucidate the mechanisms involved, mRNA expression profiles of denervated intrinsic muscles of the forepaw (IMF) and denervated biceps were determined by microarray using the rat model of OBPP where atrophy of IMF is irreversible while atrophy of biceps is reversible. Relative to contralateral control, 446 dysregulated mRNAs were detected in denervated IMF and mapped to 51 KEGG pathways, and 830 dysregulated mRNAs were detected in denervated biceps and mapped to 52 KEGG pathways. In denervated IMF, 10 of the pathways were related to muscle regulation; six with down-regulated and one with up-regulated mRNAs. The remaining three pathways had both up- and down-regulated mRNAs. In denervated biceps, 13 of the pathways were related to muscle regulation, six with up-regulated and seven with down-regulated mRNAs. Five of the pathways with up-regulated mRNAs were related to regrowth and differentiation of muscle cells. Among the 23 pathways with dysregulated mRNAs, 13 were involved in regulation of neuromuscular junctions. Our results demonstrated that mRNAs expression characteristics in irreversibly atrophic denervated IMF were different from those in reversibly atrophic denervated biceps; dysregulated mRNAs in IMF were associated with inactive pathways of muscle regulation, and in biceps they were associated with active pathways of regrowth and differentiation. Lack of self-repair potential in IMF may be a major reason why atrophy of IMF becomes irreversible much faster than atrophy of biceps after denervation.




Similar content being viewed by others
References
Alway SE (2006) Control of muscle size during disuse, disease, and aging. Int J Sports Med 27:94–99
Bonen A, Dyck DJ, Ibrahimi A et al (1999) Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am J Physiol-Endoc Metab 276:642–649
Boome RS (2000) Traumatic branchial plexus injury. In: Gupta A, Kay SPJ, Scheker LR (eds) The growing hand: diagnosis and management of the upper extremity in children. Mosby, London, pp 653–655
Caroni P, Schneider C (1994) Signaling by insulin-like growth factors in paralyzed skeletal muscle: rapid induction of IGF1 expression in muscle fibers and prevention of interstitial cell proliferation by IGF-BP5 and IGF-BP4. J Neurosci 14:3378–3388
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Chuang DC, Mardini S, Ma HS (2005) Surgical strategy for infant obstetrical brachial plexus palsy: experiences at Chang Gung Memorial Hospital. Plast Reconstr Surg 116:132–42; discussion 43–44
Cisternas P, Henriquez JP, Brandan E et al (2014) Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol 49:574–589
Da Cruz S, Parone PA, Lopes VS et al (2012) Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab 15:778–786
Dionyssiou MG, Salma J, Bevzyuk M et al (2013) Krüppel-like factor 6 (KLF6) promotes cell proliferation in skeletal myoblasts in response to TGFβ/Smad3 signaling. Skelet Muscle 3:7
Fanzani A, Conraads VM, Penna F et al (2012) Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle 3:163–179
Flück M, Carson JA, Gordon SE et al (1999) Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol-Cell Physiol 277:C152–C162
Fu AKY, Cheung ZH, Ip NY (2008) β-catenin in reverse action. Nat Neurosci 11:244–246
Getchell TV, Peng X, Green CP et al (2004) In silico analysis of gene expression profiles in the olfactory mucosae of aging senescence-accelerated mice. J Neurosci Res 77:430–452
Jordán-Álvarez S, Fouquet W, Sigrist SJ et al (2012) Presynaptic PI3K activity triggers the formation of glutamate receptors at neuromuscular terminals of Drosophila. J Cell Sci 125:3621–3629
Kelley D, Mitrakou A, Marsh H et al (1988) Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Investig 81:1563
Klinedinst S, Wang X, Xiong X et al (2013) Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. J Neurosci 33:12764–12778
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Ma J, Shen J, Garrett JP et al (2007) Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. J Orthop Res 25:1498–1505
Machida M, Takeda K, Yokono H et al (2012) Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol 227:1569–1576
Ong LL, Kim N, Mima T et al (1998) Trabecular myocytes of the embryonic heart require N-cadherin for migratory unit identity. Dev Biol 193:1–9
Pan F, Chen L, Ding F et al (2015) Expression profiles of MiRNAs for intrinsic musculature of the forepaw and biceps in the rat model simulating irreversible muscular atrophy of obstetric brachial plexus palsy. Gene 565:268–274
Pollari E, Goldsteins G, Bart G et al (2014) The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front Cell Neurosci 8:131
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102:2389–2397
Prokop A, Landgraf M, Rushton E et al (1996) Presynaptic development at the Drosophila neuromuscular junction: assembly and localization of presynaptic active zones. Neuron 17:617–626
Rawson JM, Lee M, Kennedy EL et al (2003) Drosophila neuromuscular synapse assembly and function require the TGF-b type I receptor saxophone and the transcription factor Mad. J Neurobiol 55:134–150
Redshaw Z, Sweetman D, Loughna PT (2014) The effects of age upon the expression of three miRNAs in muscle stem cells isolated from two different porcine skeletal muscles. Differentiation 88:117–123
Röder IV, Petersen Y, Choi KR et al (2008) Role of Myosin Va in the plasticity of the vertebrate neuromuscular junction in vivo. Plos One. doi:10.1371/journal.pone.0003871
Roganovic Z (2005) Missile-caused complete lesions of the peroneal nerve and peroneal division of the sciatic nerve: results of 157 repairs. Neurosurgery 57:1201–1212
Rouger K, Le Cunff M, Steenman M et al (2002) Global/temporal gene expression in diaphragm and hindlimb muscles of dystrophin-deficient (mdx) mice. Am J Physiol-Cell Physiol 283:C773–C784
Sanes JR, Lichtman JW (1999) Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22:389–442
Schiaffino S, Dyar KA, Ciciliot S et al (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280:4294–4314
Schieber MH, Santello M (2004) Hand function: peripheral and central constraints on performance. J Appl Physiol 96:2293–2300
Tanaka T, Yamamoto J, Iwasaki S et al (2003) Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci 100:15924–15929
Wagenmakers AJ (1997) Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev 26:287–314
Wei Y, Chen K, Whaley-Connell AT et al (2008) Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol-Regul, Integr Comp Physiol 294:R673–R680
Wu H, Naya FJ, McKinsey TA et al (2000) MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J 19:1963–1973
Wu JX, Chen L, Ding F et al (2013) A rat model study of atrophy of denervated musculature of the hand being faster than that of denervated muscles of the arm. J Muscle Res Cell Motil 34:15–22
Yatsenko AS, Shcherbata HR (2014) Drosophila miR-9a targets the ECM receptor Dystroglycan to canalize myotendinous junction formation. Dev Cell 28:335–348
Yeow K, Cabane C, Turchi L et al (2002) Increased MAPK signaling during in vitro muscle wounding. Biochem Biophys Res Commun 293:112–119
Zafeiriou DI (2004) Primitive reflexes and postural reactions in the neurodevelopmental examination. Pediatr Neurol 31:1–8
Acknowledgments
This research project was funded by the Chinese 973 Project Planning (2014CB542203).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Jx., Chen, L., Ding, F. et al. mRNA expression characteristics are different in irreversibly atrophic intrinsic muscles of the forepaw compared with reversibly atrophic biceps in a rat model of obstetric brachial plexus palsy (OBPP). J Muscle Res Cell Motil 37, 17–25 (2016). https://doi.org/10.1007/s10974-016-9442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-016-9442-8