Abstract
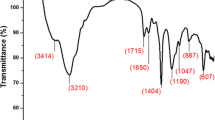
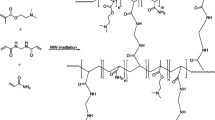
Hydrogel of poly(acrylamide-co-sodium acrylate) may be used to remove water from liquid fuels. Synthesis, characterization, and thermal degradation kinetic are reported in this article. The hydrogel was obtained by free radical polymerization, using the acrylamide and sodium acrylate monomers. The conversion of C=C double bond of the monomers and identification of the functional groups in the structure of the polymer were confirmed by Fourier transform infrared spectroscopy. The swelling capacity in distilled water was 105.68 (g water g−1 dry hydrogel). Kinetic parameters were determined by Korsmeyer–Peppas equation, with a diffusional exponent of 0.6098 and a kinetic constant of 3.7652. TG and DSC experiments were carried out to verify the thermal stability and phase transitions, respectively. Hydrogels showed high thermal stability, with degradation at 463.15–843.15 K. The glass transition temperature was approximately 383.15 K. The thermal degradation kinetics revealed that the activation energy was approximately 500,000 J mol−1. The experimental values obtained using the Vyazovkin method were compared with the models proposed by Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose. The Kissinger method exhibited the best performance describing the activation energy values for the studied conversion range.













Similar content being viewed by others
References
Kalaleh HA, Tally M, Atassi Y. Preparation of poly(sodium acrylate-co-acrylamide) superabsorbent copolymer via alkaline hydrolysis of acrylamide using microwave irradiation. 2015. https://www.researchgate.net/publication/272194220_Preparation_of_polysodium_acrylate-co-acrylamide_superabsorbent_copolymer_via_alkaline_hydrolysis_of_acrylamide_using_microwave_irradiation. Accessed 15 Nov 2019.
Pourjavadi A, Soleyman R, Bardajee GR, Ghavami S. Novel superabsorbent hydrogel based on natural hybrid backbone: optimized synthesis and its swelling behavior. Bull Korean Chem Soc. 2009;30:2680–6.
Raju MP, Raju KM. Design and synthesis of superabsorbent polymers. J Appl Polym Sci. 2001;80:2635–9.
Dai L, Cheng T, Wang Y, Wang B. A self-assembling guar gum hydrogel for efficient oil/water separation in harsh environments. Sep Purif Technol. 2019;225:129–35.
Fregolente PBL, Gonçalves HL, Maciel MRW, Fregolente LV. Swelling degree and diffusion parameters of poly(sodium acrylate-co-acrylamide) hydrogel for removal of water content from biodiesel. Chem Eng Trans. 2018;65:445–50.
Santos FB, Perez ID, Fregolente PB, Vieira MGA, Fregolente LV, Maciel MRW. Estudo da remoção de água em biodiesel comercial utilizando hidrogel de acrilamida (aam) co-polimerizado com acrilato de sódio e ácido acrílico (In Portuguese). Congresso Brasileiro de Sistemas Particulados. 2019. Study of the removal of water in commercial biodiesel using hydrogel of acrylamide (AAM) co-polymerized with sodium acrylate and acrylic acid. Accessed 15 Nov 2019.
Gonçalves HL, Fregolente PBL, Maciel MRW, Fregolente LV. Formulation of hydrogels for water removal from diesel and biodiesel. Sep Sci Technol. 2020. https://doi.org/10.1080/01496395.2019.1709506.
Jeyapriya M, Meenarathi B, Tung KL, Anbarasan R. Synthesis, characterization and applications of nano-Ag-tagged poly(ε-caprolactone-block-tetrahydrofuran). Polym Bull. 2019;77:2631–57.
Ng HM, Saidi NM, Omar FS, Kasi R. Thermogravimetric analysis of polymers. In: Mark HF, editor. Encyclopedia of polymer science and technology. New York: Wiley; 2018. https://doi.org/10.1002/0471440264.pst667.
John J, Rugmini S, Nair BS. Effect of the substituent on the thermal stability of spiroborate esters of curcumin. J Therm Anal Calorim. 2019;130:3537–47.
Huang Z, Wang XJ, Lu T, Nong DD, Gao XY, Zhao JX, Wei MY, Teng LJ. Isoconversional kinetic analysis of thermal decomposition of 1-butyl-3-methylimidazolium hexafluorophosphate under inert nitrogen and oxidative air atmospheres. J Therm Anal Calorim. 2020;140:695–712.
Trache D, Abdelaziz A. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-016-5962-0.
Alsayed Z, Awad R, Badawi BS. Thermo-mechanical properties of high density polyethylene with zinc oxide as a filler. Iran Polym J. 2020;29:309–20.
Byrn RS, Zografi G, Chen XS. Differential scanning calorimetry and thermogravimetric analysis. 1st ed. London: Wiley; 2017.
Trache D, Abdelaziz A. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-016-5962-0.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Comput Sci. 1996;36:42–5.
Xue Z, Wang S, Lin L, Chen L, Liu M, Feng L, Jiang LA. Novel super hydrophilic and underwater super oleophobic hydrogel-coated mesh for oil/water Separation. Adv Mater. 2011;23:4270–3.
Kajjari PB, Manjeshwar LS, Aminabhavi TM. Semi-interpenetrating polymer network hydrogel blend microspheres of gelatin and hydroxyethyl cellulose for controlled release of theophylline. Ind Eng Chem Res. 2011;50:7833–40.
Jabbari E, Nozari S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur Polym J. 2000;36:2685–92.
Vyazovkin S, Rives V, Schick C. Making impact in thermal sciences: overview of highly cited papers published in Thermochimica Acta. Thermochim Acta. 2010;500:1–5.
Vyazovkin S, Wight CA. Kinetics of thermal decomposition of cubic ammonium perchlorate. Chem Mater. 1999;11:3386–93.
Feng L, Xang H, Dong X, Lei H, Chen D. pH-sensitive polymeric particles as smart carriers for rebar inhibitors delivery in alkaline condition: research article. J Appl Polym Sci. 2017. https://doi.org/10.1002/app.45886.
Karthikeyan S, Anandan C, Subramaniam J, Sekaran G. Characterization of iron impregnated polyacrylamide catalyst and its application to the treatment of municipal wastewater. RSC Adv. 2013. https://doi.org/10.1039/c3ra41733f.
El-Din HMN, Alla SGA, El-Naggar AWM. Radiation synthesis and characterization of hydrogels composed of poly(vinyl alcohol) and acrylamide mixtures. J Macromol Sci Part A Pure Appl Chem. 2007;44:47–54.
Dumitrescu AM, Lisa G, Iordan A, Tudorache F. Ni ferrite highly organized as humidity sensors. Mater Chem Phys. 2015;156:170–9.
Nesrinne S, Djamel A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab J Chem. 2017;10:539–47.
Sadeghi M, Hosseinzadeh H. Synthesis of starch-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with salt and pH-responsiveness properties as a drug delivery system. J Bioact Compat Polym. 2008;23:381–404.
Aouada FA, Muniz EC, Vaz CMP, Mattoso LHC. Correlation between parameters of swelling kinetic with network and hydrophilic characteristics of polyacrylamide and methylcellulose hydrogels. Quim Nova. 2008;32:1482–90.
Sultana S. Swelling and physico-mechanical properties of synthesized sodium polyacrylate hydrogels. Int J Adv Res. 2017;5:84–92.
Malana MA, Aftab F, Batool SR. Synthesis and characterization of stimuli-responsive hydrogel based on starch and methyl-3-aminocrotonate: swelling and degradation kinetics. Polym Bull. 2019;76:3073–92.
Fu X, Chen T, Xu X, Zhang Y. Thermal stability and decomposition kinetics of poly(methacryloyloxyethyl trimethyl ammonium chloride-co-acrylamide). J Macromol Sci Part B Phys. 2019;2348:659–72.
Canevarolo SV. Técnicas de caracterização de polímeros. Artiibcr. Brazil; 2003. p. 209–29.
Biliaderis CG, Lazaridou A, Arvanitoyannis L. Glass transition and physical properties of polyol-plasticized pullulan-starch blends at low moisture. Carbohydr Polym. 1999;40:29–47.
Maurer JJ, Eustace DJ, Ratcliffe CT. Thermal characterization of poly (acrylic acid). Macromolecules. 1987;2:196–202.
Huang Y, Yu H, Xiao C. pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: dwelling and in vitro drug release. Carbohydr Polym. 2007;69:774–83.
Sigma Aldrich. Polymer properties. https://www3.nd.edu/~hgao/thermal_transitions_of_homopolymers.pdf. Accessed 15 Nov 2019.
Alves TVG, Tavares EJM, Aouada FM, Negrão CAB, Oliveira MEC, Júnior APD, Costa CEF, Júnior JOCS, Costa RMR. Thermal analysis characterization of PAAm-co-MC hydrogels. J Therm Anal Calorim. 2011;106:717–24.
Horia M, El-Din N, Alla SGA, El-Naggar AWM. Radiation synthesis and characterization of hydrogels composed of poly(vinyl alcohol) and acrylamide mixtures. J Macromol Sci. 2007;44:47–54.
Kitahara Y, Okuyama K, Ozawa K, Takuya S, Takahashi S, Toshihiro F. Thermal decomposition of acrylamide from polyacrylamide. J Therm Anal Calorim. 2012. https://doi.org/10.1007/s10973-012-2544-7.
Acknowledgements
The authors gratefully acknowledge the Grants #2018/16269-2, #2016/18546-8, #2017/12120-1, and #2015/20630-4, São Paulo Research Foundation (FAPESP), the National Institute of Science and Technology in Biofabrication (BIOFABRIS), and the National Council of Technological and Scientific Development (CNPq) by financial support.
Funding
This research was supported by São Paulo Research Foundation (FAPESP) (Grants #2018/16269-2, #2016/18546-8, #2017/12120-1, and #2015/20630-4), the National Institute of Science and Technology in Biofabrication (BIOFABRIS), and the National Council of Technological and Scientific Development (CNPq).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: FBdosS. Methodology: FBdosS. Formal analysis and investigation: FBdosS, NTM, and MIRBS. Writing—original draft preparation: FBdosS and NTM. Writing—review and editing: FBdosS, NTM, MRWM, and LVF. Funding acquisition: MRWM and LVF. Resources: FBdosS. Supervision: MRWM and LVF.
Corresponding author
Ethics declarations
Conflicts of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, F.B., Miranda, N.T., Schiavon, M.I.R.B. et al. Thermal degradation kinetic of poly(acrylamide-co-sodium acrylate) hydrogel applying isoconversional methods. J Therm Anal Calorim 146, 2503–2514 (2021). https://doi.org/10.1007/s10973-020-09899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09899-y