Abstract
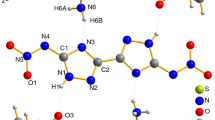
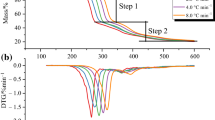
The thermal hazard of (5,6-(dicarboxylate)-pyridin-3-yl) methyl-trimethyl ammonium bromide (DPTAB) was evaluated by carrying out dynamic tests on a differential scanning calorimeter, and the thermodynamic parameters were obtained. A kinetic model was established by simulating experimental curves. The model contains two successive self-accelerating stages, and this article aims to explain the effect that these two stages have on the decomposition of DPTAB, respectively. The thermal behaviors were simulated under isothermal, adiabatic, heat-transfer limited conditions, and the results suggest that the decomposition of DPTAB is uncontrollable as it occurs, and the heat dispersed is intense. The self-accelerating decomposition temperature of DPTAB was 110.9 °C, indicating that the storage and transformation temperatures of DPTAB should not be higher than 110.9 °C. The thermal runaway system reached the maximum reaction rate at a temperature of 139.9 °C within 24 h under adiabatic conditions, thus implying that the processing temperature in industrial applications should be maintained below 139.9 °C.









Similar content being viewed by others
References
Bou-Diab L, Fierz H. Autocatalytic decomposition reactions, hazards and detection. J Hazard Mater. 2002;93:137–46.
Liu Z, Yin C, Liu Y, Fan X, Zhao F. Thermal decomposition of RDX and HMX part II: kinetic parameters and kinetic compensation effects. Chin J Explos Propellants. 2004;27(4):72–5.
Wu WX. Process for the preparation of (5,6-dicarboxy-3-pyridyl) methyl ammonium halides. J Chem Inf Model 1997; p 14.
Menges F, Gebhardt J, Rack M. 2-[(1-cyanopropyl)carbamoyl]-5-chloromethyl nicotinic acids and the use thereof in manufacturing herbicidal imidazolinones 2009; p 20.
Yuan MH, Shu CM, Kossoy AA. Kinetics and hazards of thermal decomposition of methyl ethyl ketone peroxide by DSC. Thermochim Acta. 2005;430:67–71.
Kossoy A, Benin A, Akhmetshin Y. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
Kossoy A, Sheinman IY. Evaluating thermal explosion hazard by using kinetics-based simulation approach. Process Saf Environ Prot. 2004;82:421–30.
Kossoy A, Akhmetshin Y. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26:209–20.
Rao G, Feng W, Zhang J, Wang S, Chen L, Guo Z, et al. Simulation approach to decomposition kinetics and thermal hazards of hexamethylenetetramine. J Therm Anal Calorim. 2019;135:2447–56.
Roduit B, Borgeat C, Berger B, Folly P, Alonso B, Aebischer JN, et al. Advanced kinetic tools for the evaluation of determination of thermal stability of energetic materials. J Therm Anal Calorim. 2005;80:229–36.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Lin CP, Chang CP, Chou YC, Chu YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Li H-B, Wang S-Y, Gan X-Y, Chen W-H, Chen L-P. Thermal risk analysis of benzoyl peroxide in the presence of phenol: based on the experimental and simulation approach. Thermochim Acta. 2019;681:178354.
Svoboda R, Málek J. Importance of proper baseline identification for the subsequent kinetic analysis of derivative kinetic data: part 1. J Therm Anal Calorim. 2016;124:1717–25.
Barale S, Vincent L, Sauder G, Sbirrazzuoli N. Deconvolution of calorimeter response from electrical signals for extracting kinetic data. Thermochim Acta. 2015;615:30–7.
Shen S, Jiang J, Zhang W, et al. Journal of loss prevention in the process Industries 2018;54:153–62.
Lu G, Zhang C, Chen L, Chen W, Yang T, Zhou Y. Kinetic analysis and self-accelerating decomposition temperature (SADT) of β-nitroso-α-naphthol. Process Saf Environ Prot Inst Chem Eng. 2015;95:69–76.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, XH., Tan, JS., Wei, ZY. et al. Thermal safety study of (5,6-(dicarboxylate)-pyridin-3-yl) methyl-trimethyl ammonium bromide based on decomposition kinetics. J Therm Anal Calorim 145, 2431–2439 (2021). https://doi.org/10.1007/s10973-020-09755-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09755-z