Abstract
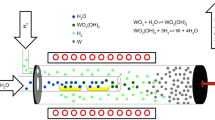
Controlling the conditions of the oxygen partial pressure and temperature to prepare the WO2.72 (W18O49) via reduction was possible through thermodynamic consideration. WO2.72 was synthesized via heating to 1073 K in 5% H2–95% Ar mixture gas flow from ammonium tungstate which was prepared by hydrothermal process. With the reducing prolonging time, the products were changed from WO2.72 to WO2 and then metal W. Thermogravimetric (TG) analysis showed ammonium tungstate decomposed completely to WO3 at 773 K. Isothermal reductions using TG analysis were carried out at 905 K, 925 K, 945 K and 973 K in 5% H2–95% Ar mixture gas flow, respectively. The whole reduction from WO3 to WO2.72 divided into three parts: initial nucleation and growth stage, final interfacial reaction stage and intermediate stage, was controlled jointly by both mechanisms. Fitting results showed that the initial stage obey the one-dimensional Avrami–Erofeev equation, the apparent activation energy was 132.7 ± 1.1 kJ mol−1 and the pre-exponent factor was 4.82 × 105 min−1; the final stage expressed by 2-dimensional interfacial reaction, the apparent activation energy was 144.0 ± 2.1 kJ mol−1 and the pre-exponent factor was 3.20 × 105 min−1.














Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (min−1)
- E :
-
Activation energy (kJ mol−1)
- \(G\left( \alpha \right)\) :
-
Integral form of kinetic mechanism function
- m :
-
Mass (mg)
- n :
-
Coefficients in the kinetic equation
- R :
-
Gas constant, 8.314 J (mol K)−1
- t :
-
Time (min)
- \(t^{\prime}\) :
-
Real reaction time (min)
- \(t_{\text{i}}\) :
-
The time at the beginning of the final stage
- T :
-
Absolute temperature (K)
- \(\alpha\) :
-
Conversion rate
- \(\alpha^{\prime}\) :
-
Real isothermal conversion rate
- \(\alpha_{\text{i}}\) :
-
Conversion rate at the beginning of the final stage
- \(\alpha_{0}\) :
-
Conversion rate of pre-reduction
References
Guo CS, Yin S, Yan M, Kobayashi M, Kakihana M, Sato T. Morphology-controlled synthesis of W18O49 nanostructures and their near-infrared absorption properties. Inorg Chem. 2012;51(8):4763–71.
Liu JW, Zheng J, Wang JL, Xu J, Li HH, Yu SH. Ultrathin W18O49 nanowire assemblies for electrochromic devices. Nano Lett. 2013;13(8):3589–93.
Xi GC, Ouyang SX, Li P, Ye JH, Ma Q, Su N, et al. Ultrathin W18O49 nanowires with diameters below 1 nm: synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angew Chem Int Ed. 2012;51(10):2395–9.
Huang ZF, Song JJ, Pan L, Lv FL, Wang QF, Zou JJ, et al. Mesoporous W18O49 hollow spheres as highly active photocatalysts. Chem Commun. 2014;50(75):10959–62.
Zhou HW, Shi YT, Dong QS, Lin J, Wang AQ, Ma TL. Surface oxygen vacancy-dependent electrocatalytic activity of W18O49 nanowires. J Phys Chem C. 2014;118(35):20100–6.
Ganesan R, Perelshtein I, Gedanken A. Biotemplated synthesis of single-crystalline W18O49@C core–shell nanorod and its capacitance properties. J Phys Chem C. 2008;112(6):1913–9.
Karim NA, Kamarudin SK, Shyuan LK, Yaakob Z, Daud WRW, Kadhum AAH. Study on the electronic properties and molecule adsorption of W18O49 nanowires as a catalyst support in the cathodes of direct methanol fuel cells. J Power Sources. 2015;288:461–72.
Sun Y, Wang W, Qin JW, Zhao D, Mao BG, Xiao Y, et al. Oxygen vacancy-rich mesoporous W18O49 nanobelts with ultrahigh initial Coulombic efficiency toward high-performance lithium storage. Electrochim Acta. 2016;187:329–39.
Jeon S, Yong KJ. Direct synthesis of W18O49 nanorods from W2N film by thermal annealing. Nanotechnology. 2007. https://doi.org/10.1088/0957-4484/18/24/245602.
Agiral A, Gardeniers JGE. Synthesis and atmospheric pressure field emission operation of W18O49 nanorods. J Phys Chem C. 2008;112(39):15183–9.
Guo CS, Yin S, Huang YF, Dong Q, Sato T. Synthesis of W18O49 nanorod via ammonium tungsten oxide and its interesting optical properties. Langmuir. 2011;27(19):12172–8.
Zhou J, Ding Y, Deng SZ, Gong L, Xu NS, Wang ZL. Three-dimensional tungsten oxide nanowire networks. Adv Mater. 2005;17(17):2107–10.
Yella A, Tahir MN, Meuer S, Zentel R, Berger R, Panthofer M, et al. Synthesis, characterization, and hierarchical organization of tungsten oxide nanorods: spreading driven by marangoni flow. J Am Chem Soc. 2009;131(48):17566–75.
Shi SL, Xue XY, Feng P, Liu YG, Zhao H, Wang TH. Low-temperature synthesis and electrical transport properties of W18O49 nanowires. J Cryst Growth. 2008;310(2):462–6.
Wriedt HA. The OW (oxygen-tungsten) system. Bull Alloy Phase Diagr. 1989;10(4):368–84.
Wang YZ, Li F, Yan BJ, Fan TF, Xu MD, Gong HY. Kinetic study on preparation of substoichiometric titanium oxide via carbothermal process. J Therm Anal Calorim. 2015;122(2):635–44.
Kalpakli AO, Arabaci A, Kahruman C, Yusufoglu I. Thermal decomposition of ammonium paratungstate hydrate in air and inert gas atmospheres. Int J Refract Met Hard Mater. 2013;37:106–16.
Szilagyi IM, Madarasz J, Pokol G, Hange F, Szalontai G, Varga-Josepovits K, et al. The effect of K + ion exchange on the structure and thermal reduction of hexagonal ammonium tungsten bronze. J Therm Anal Calorim. 2009;97(1):11–8.
Xu T, Jian ZY, Chang FG, Zhuo LC, Zhang T. Isothermal crystallization kinetics of Fe75Cr5P9B4C7 metallic glass with cost-effectiveness and desirable merits. J Therm Anal Calorim. 2018;133(3):1309–15.
Gualtieri AF, Ricchi A, Gualtieri ML, Maretti S, Tamburini M. Kinetic study of the drying process of clay bricks. J Therm Anal Calorim. 2016;123(1):153–67.
Guo L, Zhao HB, Wang K, Mei DF, Ma ZJ, Zheng CG. Reduction kinetics analysis of sol–gel-derived CuO/CuAl2O4 oxygen carrier for chemical looping with oxygen uncoupling. J Therm Anal Calorim. 2016;123(1):745–56.
Acknowledgements
This work is financially supported by National Natural Science Foundation of China (NSFC 51472009, 51534001, 51172007) and Beijing Municipal High Level Innovative Team Building Program (IDHT20170502). Authors express thanks to large-scale instrument and equipment sharing forum of Beijing University of Technology for providing the excellent analysis conditions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, D., Wang, Y., Li, F. et al. Kinetic study on preparation of substoichiometric tungsten oxide WO2.72 via hydrogen reduction process. J Therm Anal Calorim 137, 389–397 (2019). https://doi.org/10.1007/s10973-018-7966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7966-4