Abstract
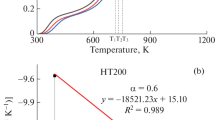
The cleavage behavior of covalent bonds in Xilinguole (XLGL) lignite and changes in chemical structure of lignite and its chars during low-temperature pyrolysis were investigated by thermogravimetric (TG) analysis and Fourier-transform infrared (FTIR) spectroscopy. Based on the TG and differential thermogravimetric (DTG) analysis results, the cleavage of different types of chemical bonds in lignite occurred mainly at four certain temperatures, 170 °C, 376 °C, 432 °C, and 521 °C. The latter three were selected as the final pyrolysis temperatures of chars evaluated in this study. The FTIR analysis results indicate that thermal treatment increased the relative content of two and three adjacent H deformation structures but decreased that of four adjacent H deformation structure. This was caused by the cleavage of Cal–Cal and Car–Cal bonds. The oxygen-containing functional groups in lignite are dominated by C–O and C–OH groups with a lower chemical reactivity than C=O–C and conjugated C=O groups. Moreover, XLGL lignite has the highest ratio of CH2/CH3 which declines with increasing temperature, indicating the decrease in the length of aliphatic chains and increase in the degree of branching of aliphatic side chains. This change mainly resulted from the cleavage of Cal–O, Cal–Cal, and Car–Cal bonds. Furthermore, XLGL lignite and its chars contain five specific hydrogen bonds: OH–N, cyclic OH, OH–ether O, OH–OH, and OH–π hydrogen bonds. The relative content of OH–OH hydrogen bond was the highest, indicating that OH–OH hydrogen bond has the highest thermal stability.










Similar content being viewed by others
References
Xu F, Pan S, Liu C, Zhao D, Liu H, Wang Q, et al. Construction and evaluation of chemical structure model of Huolinhe lignite using molecular modeling. RSC Adv. 2017;7(66):41512–9. https://doi.org/10.1039/c7ra07387a.
Li F, Fan LS. Clean coal conversion processes - progress and challenges. Energy Environ Sci. 2008;1(2):248–67.
Karthikeyan M, Zhonghua W, Mujumdar AS. Low-rank coal drying technologies-current status and new developments. Dry Technol. 2009;27(3):403–15.
Ahmed II, Gupta AK. Experiments and stochastic simulations of lignite coal during pyrolysis and gasification. Appl Energy. 2013;102(2):355–63.
Han Y, Yin F, Li X, et al. A review on water in low rank coals: The existence, interaction with; coal structure and effects on coal utilization. Fuel Process Technol. 2013;106(2):9–20.
Liu P, Zhang D, Wang L, Yang Z, Pan T, Lu X. The structure and pyrolysis product distribution of lignite from different sedimentary environment. Appl Energy. 2016;163:254–62.
Liu M, Qin Y, Yan H, Han X, Chong D. Energy and water conservation at lignite-fired power plants using drying and water recovery technologies. Energy Convers Manag. 2015;105:118–26.
Chen J, Lin M, Cai J, Yin H, Song X, Li A. Thermal characteristics and kinetics of refining and chemicals wastewater, lignite and their blends during combustion. Energy Convers Manag. 2015;100:201–11.
Guan Y, Ma Y, Zhang K, Chen H, Xu G, Liu W, et al. Co-pyrolysis behaviors of energy grass and lignite. Energy Convers Manag. 2015;93:132–40.
Lu D, Zhou Z, Dai Z, Yu G. Effects of coal drying on the pyrolysis and in situ gasification characteristics of lignite coals. Appl Energy. 2015;155:660–70.
Xu Y, Zhang Y, Wang Y, Zhang G, Chen L. Gas evolution characteristics of lignite during low-temperature pyrolysis. J Anal Appl Pyrol. 2013;104:625–31. https://doi.org/10.1016/j.jaap.2013.05.004.
Xu Y, Zhang Y, Zhang G, Guo Y. Low temperature pyrolysates distribution and kinetics of Zhaotong lignite. Energy Convers Manag. 2016;114:11–9.
Zhang D, Liu P, Lu X, Wang L, Pan T. Upgrading of low rank coal by hydrothermal treatment: coal tar yield during pyrolysis. Fuel Process Technol. 2016;141:117–22.
Gong X, Wang Z, Deng S, Li S, Song W, Lin W. Impact of the temperature, pressure, and particle size on tar composition from pyrolysis of three ranks of Chinese coals. Energy Fuels. 2014;28(8):4942–8.
Kabir KB, Hein K, Bhattacharya S. Process modelling of dimethyl ether production from Victorian brown coal-Integrating coal drying, gasification and synthesis processes. Comput Chem Eng. 2013;48(48):96–104.
Bhattacharya S, Kabir KB, Hein K. Dimethyl ether synthesis from Victorian brown coal through gasification—current status, and research and development needs. Prog Energy Combust Sci. 2013;39(6):577–605.
Zeng X, Wang Y, Yu J, Wu S, Han J, Xu S, et al. Gas upgrading in a downdraft fixed-bed reactor downstream of a fluidized-bed coal pyrolyzer. Energy Fuels. 2011;25(11):5242–9.
Sun M, Chen J, Dai XM, Zhao XL, Liu K, Ma XX. Controlled separation of low temperature coal tar based on solvent extraction-column chromatography. Fuel Process Technol. 2015;136:41–9.
Mohan D, Pittman CU, Steele PH. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels. 2006;20(3):848–89.
Li Y, Wang ZH, Huang ZY, Liu JZ, Zhou JH, Cen KF. Effect of pyrolysis temperature on lignite char properties and slurrying ability. Fuel Process Technol. 2015;134:52–8.
Wang K, Deng J, Zhang YN, Wang CP. Kinetics and mechanisms of coal oxidation mass gain phenomenon by TG–FTIR and in situ IR analysis. J Therm Anal Calorim. 2018;132(1):591–8. https://doi.org/10.1007/s10973-017-6916-x.
Naktiyok J, Bayrakçeken H, Özer AK, Gülaboğlu MŞ. Investigation of combustion kinetics of Umutbaca-lignite by thermal analysis technique. J Therm Anal Calorim. 2017;129(1):531–9. https://doi.org/10.1007/s10973-017-6149-z.
Liu H, Gong S, Jia C, Wang Q. TG-FTIR analysis of co-combustion characteristics of oil shale semi-coke and corn straw. J Therm Anal Calorim. 2016;127(3):2531–44. https://doi.org/10.1007/s10973-016-5739-5.
Shi L, Liu Q, Guo X, Wu W, Liu Z. Pyrolysis behavior and bonding information of coal—a TGA study. Fuel Process Technol. 2013;108:125–32. https://doi.org/10.1016/j.fuproc.2012.06.023.
Cheng J, Zhang Y, Wang T, Norris P, Chen WY, Pan WP. Thermogravimetric–Fourier-transform infrared spectroscopy–gas chromatography/mass spectrometry study of volatile organic compounds from coal pyrolysis. Energy Fuels. 2017;31(7):7042–51.
He Q, Wan K, Hoadley A, Yeasmin H, Miao Z. TG–GC–MS study of volatile products from Shengli lignite pyrolysis. Fuel. 2015;156:121–8. https://doi.org/10.1016/j.fuel.2015.04.043.
Meng F, Yu J, Tahmasebi A, Han Y, Zhao H, Lucas J. Characteristics of chars from low-temperature pyrolysis of lignite. Energy Fuels. 2013;28(1):275–84. https://doi.org/10.1021/ef401423s.
Wang JP, Li GY, Guo R, Li AQ, Liang YH. Theoretical and experimental insight into coal structure: establishing a chemical model for Yuzhou lignite. Energy Fuels. 2016;31(1):124–32. https://doi.org/10.1021/acs.energyfuels.6b01854.
Wang YG, Wei XY, Wang SK, Li ZK, Li P, Liu FJ, et al. Structural evaluation of Xiaolongtan lignite by direct characterization and pyrolytic analysis. Fuel Process Technol. 2016;144:248–54. https://doi.org/10.1016/j.fuproc.2015.12.034.
Ibarra J, Moliner R, Bonet AJ. FT-I.R. investigation on char formation during the early stages of coal pyrolysis. Fuel. 1994;73(6):918–24.
Han F, Zhang YG, Meng AH, Li QH. FTIR analysis of Yunnan Lignite. J China Coal Soc. 2014;39(11):2293–9.
Yu S, Zhu YM, Wu LI. Structure evolution of oxygen functional groups in Dongsheng long flame coal by 13C-NMR and FT-IR. J Fuel Chem Technol. 2015;43(5):519–29.
Wang Q, Cui D, Wang P, Bai J, Wang Z, Liu B. A comparison of the structures of > 300°C fractions in six Chinese shale oils obtained from different locations using 1H NMR, 13C NMR and FT-IR analyses. Fuel. 2018;211:341–52. https://doi.org/10.1016/j.fuel.2017.09.071.
Wang Q, Ye JB, Yang HY, Liu Q. Chemical composition and structural characteristics of oil shales and their kerogens using Fourier transform infrared (FTIR) spectroscopy and solid-state 13C nuclear magnetic resonance (NMR). Energy Fuels. 2016;30(8):6271–80. https://doi.org/10.1021/acs.energyfuels.6b00770.
Meng F, Tahmasebi A, Yu J, Zhao H, Han Y, Lucas J, et al. Low-temperature oxidation characteristics of lignite chars from low-temperature pyrolysis. Energy Fuels. 2014;28(9):5612–22. https://doi.org/10.1021/ef501004t.
Dun W, Guijian L, Ruoyu S, Xiang F. Investigation of structural characteristics of thermally metamorphosed coal by FTIR spectroscopy and X-ray diffraction. Energy Fuels. 2013;27(10):5823–30. https://doi.org/10.1021/ef401276h.
He X, Liu X, Nie B, Song D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel. 2017;206:555–63. https://doi.org/10.1016/j.fuel.2017.05.101.
Xiong G, Li Y, Jin L, Hu H. In situ FT-IR spectroscopic studies on thermal decomposition of the weak covalent bonds of brown coal. J Anal Appl Pyrol. 2015;115:262–7. https://doi.org/10.1016/j.jaap.2015.08.002.
Georgakopoulos A, Iordanidis A, Kapina V. Study of low rank Greek coals using FTIR spectroscopy. Energy Sources. 2003;25(10):995–1005.
Geng W, Nakajima T, Takanashi H, Ohki A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel. 2009;88(1):139–44.
Hämäläinen JP, Aho MJ. Conversion of fuel nitrogen through HCN and NH3 to nitrogen oxides at elevated pressure. Fuel. 1996;75(12):1377–86.
Thomas KM. The release of nitrogen oxides during char combustion. Fuel. 1997;76(6):457–73.
Acknowledgements
This work was funded through the support coming from the National Natural Science Foundation of China (No. 51676032 and 5176034) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT-17R19).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Liu, Y., Xu, F. et al. Insights on structural characteristic of Xilinguole lignite chars from low-temperature pyrolysis. J Therm Anal Calorim 136, 1631–1643 (2019). https://doi.org/10.1007/s10973-018-7784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7784-8