Abstract
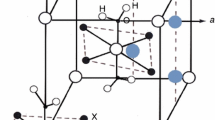
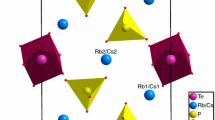
Thermal properties and phase transformations of Rb2HPO4·2H2O have been investigated by differential scanning calorimetry and thermogravimetry and compared with ones of Cs2HPO4·2H2O. The absence of superionic phase transitions has been shown. Despite the isotypic structure, the thermal properties of Rb2HPO4·2H2O differ from those of Cs2HPO4·2H2O. The temperatures of the formation of dehydrated Rb2HPO4 and Rb4P2O7 salts are slightly lower than for cesium compounds. Rb2HPO4·2H2O was shown to decompose in two stages with the formation of Rb2HPO4 at 119 °C and Rb4P2O7 at 360 °C, respectively. The dehydration of Rb2HPO4·2H2O is characterized by non-resolved stages unlike Cs2HPO4·2H2O. The enthalpies of Rb2HPO4·2H2O dehydration and Rb2HPO4 decomposition were determined. The proton conductivity of Rb2HPO4·2H2O and Rb2HPO4 has been investigated by ac-impedance spectroscopy for the first time. The sharp increasing of conductivity up to 10−2 S cm−1 at 75–145 °C was found caused by proton transport ways of crystalline hydrate water on the Rb2HPO4 grain boundaries. The proton conductivity of dehydrated Rb2HPO4 phase was shown to be quite low ~ 10−8 to 10−4 S cm−1 in the temperature range 100–250 °C with the activation energy of conductivity ~ 1.2 eV. The low conductivity values deal with the strong hydrogen bonds network which is consistent with the IR-spectroscopy data of vibration structure. The IR-spectrum at 22 °C has been measured and discussed in relation to the crystal structure. IR-spectroscopy characteristics of Rb2HPO4·2H2O differ by strong shifts of some P–O bands of PO3(OH) tetrahedra due to the shorter interatomic distances in comparison with Cs2HPO4·2H2O.







Similar content being viewed by others
References
Baranov AI. Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Review. Crystallogr Rep. 2003;48(6):1012–37.
Qing G, Kikuchi R, Takagaki A, Sugawara T, Ted Oyama ST. CsH2PO4/polyvinylidene fluoride composite electrolytes for intermediate temperature fuel cells. J Electrochem Soc. 2014;161(4):F451–7.
Haile SM, Chisholm CRI, Sasaki K, Boysen DA, Uda T. Solid acid proton conductors: from laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007;134:17–39.
Li Z. Impedance analysis and protonic conduction mechanism in RbH2PO4/SiO2 composite systems. Electrochim Acta. 2010;55:7298–304.
Ponomareva VG, Lavrova GV, Simonova LG. Effect of SiO2 morphology and pores size on the proton nanocomposite electrolytes. Solid State Ion. 1999;119:295–9.
Ponomareva VG, Shutova ES. High-temperature behavior of CsH2PO4 and CsH2PO4–SiO2 composites. Solid State Ion. 2007;178:729–34.
Ponomareva VG, Lavrova GV, Simonova LG. The influence of heterogeneous dopant porous structure on the properties of the protonic solid electrolyte in the CsHSO4–SiO2 system. Solid State Ion. 1999;118:317–23.
Ponomareva VG, Lavrova GV. Controlling the proton transport properties of solid acids via structural and microstructural modification. J Solid State Electrochem. 2011;15:213–21.
Martsinkevich VV, Ponomareva VG. Double salts Cs1−xMxH2PO4 (M = Na, K, Rb) as proton conductors. Solid State Ion. 2012;225:236–40.
Ikeda A, Kitchaev DA, Haile SM. Phase behavior and superprotonic conductivity in the Cs1−xRbxH2PO4 and Cs1−xKxH2PO4 systems. J Mater Chem A. 2014;2:204–14.
Ponomareva VG, Bagryantseva IN. Superprotonic CsH2PO4–CsHSO4 solid solutions. Inorg Mater. 2012;48(2):189–96.
Baranov AI, Shuvalov LA, Schagina NM. Superion conductivity and phase transitions in CsHSO4 and CsHSeO4 crystals. JETP Lett. 1982;36:459–62.
Boysen DA. Superprotonic solid acids: structure, properties and applications. degree of doctor of philosophy. Pasadena: California Institute of Technology; 2004. p. 172.
Taninouchi YK, Uda T, Awakura Y, Ikeda A, Haile SM. Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260°C. J Mater Chem. 2007;17(30):3182–9.
Rapoport E, Clark JB, Richter PW. High-pressure phase relations of RbH2PO4, CsH2PO4, and KD2PO4. J Solid State Chem. 1978;24(3–4):423–33.
Dostál K, Fukanová P, Mezník L. Thermische Kondensation von Alkalihydrogenphosphaten und –hydrogenarsenaten. Collect Czech Chem Commun. 1973;38:667–72.
Nirsha BM, Gudinitsa AN, Efremov VA, Fakeev AA, Jadanov BV, Olikova VA. Thermal dehydration of Rb2HPO4·2H2O. Zh Neorg Khim. 1981;26(10):2606–11.
Nirsha BM, Gudinitsa AN, Efremov VA, Fakeev AA. Study of thermal-decomposition of cesium disubstituted ortho-phosphate sesquihydrate. Zh Neorg Khim. 1983;28(4):840–3.
Stoeger B, Weil M. The isotypic hydrogen phosphate and arsenate dihydrates M2HXO4·2H2O (M = Rb, Cs; X = P, As). Acta Crystallogr. 2014;C70:7–11.
Catti M, Ferraris G, Franchini-Angela M. The crystal structure of Na2HPO4·2H2O. Competition between coordination and hydrogen bonds. Acta Crystallogr. 1977;B33:3449–52.
Lazrak R, Nadifiyine M, Tanouti B, Mokhlisse A, Guion J, Tenu SR, Berthet J, Counioux JJ. Etude par conductimétrie des isothermes 40 °C des systèmes ternaires: M2HPO4-M2′HPO4-H2O (M, M′ = Na, K, NH4). J Solid State Chem. 1995;119:68–75.
Soboleva LV, Voloshin AE. Me2O–P2O5–H2O solubility phase diagrams and growth of MeH2PO4 single crystals (Me = Li, Na, K, Rb, Cs, NH4). Crystallogr Rep. 2004;49(4):693–7.
Lavrova GV, Bulina NV, Min’kov VS, Matvienko AA. Structure and thermal decomposition of Cs2HPO4·2H2O. Russ J Inorg Chem. 2016;61(3):284–90.
Ponomareva VG, Bagryantseva IN, Lavrova GV. Proton conductivity and spectral data of Cs2HPO4·2H2O. Russ J Electrochem. 2017;53(6):636–40.
Baran J, Lis T, Ratajczak H. Structure and polarized IR spectra of the K2HPO4·3H2O crystal. J Mol Struct. 1989;195:159–74.
Catti M, Ferraris G, Ivaldi G. Disorder of HPO4 2− and of hydrogen bonds in the structure of β-Na2HPO4·12H2O. Acta Crystallogr. 1978;B34:369–73.
Baur WH, Khan AA. On the crystal chemistry of salt hydrates. VI. The crystal structures of disodium hydrogen orthoarsenate heptahydrate and of disodium hydrogen orthophosphate heptahydrate. Acta Crystallogr. 1970;B26:1584–96.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;32:751–67.
Sheludyakova LA, Afanasieva VA, Podberezskaya NV, Mironov YuI. Spectral and structural analysis of sodium hydrophosphates and hydroarsenates. Russ J Struct Chem. 1999;40:869–72.
Acknowledgements
The authors thank their colleagues Dr. N.V. Bulina, Dr. K.B. Gerasimov, Dr. E.A. Losev and Dr. A.A. Matvienko for the help in performance of experiments on X-ray diffraction patterns, differential scanning calorimetry, IR-spectroscopy, scanning electron microscopy and energy-dispersive X-ray spectroscopy. This research was carried out within the State Assignment to ISSCM SB RAS (Project 0301-2018-0002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaydamaka, A.A., Bagryantseva, I.N. & Ponomareva, V.G. Thermal properties, proton conductivity and vibration study of Rb2HPO4·2H2O. J Therm Anal Calorim 133, 1121–1127 (2018). https://doi.org/10.1007/s10973-018-7402-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7402-9