Abstract
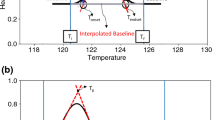
Differential scanning calorimetry (DSC), isothermal stress testing–Fourier transform infrared spectroscopy (IST–FTIR), isothermal stress testing–high-performance liquid chromatography, and powder X-ray diffraction (PDRX) were used as screening techniques for assessing the compatibility of tobramycin with some currently employed ophthalmic excipients. In the first phase of the study, DSC was used as a tool to detect any interaction. The absolute value of the difference between the enthalpy of the pure tobramycin melting peak and that of its melting peak in the different analyzed mixtures was chosen as a parameter of the drug–excipient interaction degree. DSC results demonstrated that benzalkonium chloride, monobasic sodium phosphate, boric acid, edetate disodium, sodium metabisulfite, thimerosal, and potassium sorbate interact with tobramycin. Taking into account these results, it could be suggested that some of the changes observed in the IST–FTIR spectra of binary blends of tobramycin and some of the excipients would account for a possible interaction between the mixture component. In this study, PDRX did not provide much information, since only tobramycin–thimerosal interactions could be detected. DSC and IST–FTIR are suitable and simple methods for the detection of potential incompatibilities between active pharmaceutical ingredient (API) and excipients.










Similar content being viewed by others
References
Bharate SS, Bharate SB, Bajaj AN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excip Food Chem. 2010;1:3–26.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J Therm Anal Calorim. 2010;99:631–7.
Ceresole R, Han Y, Rosasco MA, Orelli LR, Segall AI. Drug–excipient compatibility studies in binary mixtures of Avobenzone. J Cosmet Sci. 2013;64:317–28.
Chadha R, Bhandari S. Drug–excipient compatibility screening—role of thermoanalytical and spectroscopic techniques. J Pharm Biomed Anal. 2014;87:82–97.
McDaid FM, Barker SA, Fitzpatrick S, Petts CR, Craig DQM. Further investigations into the use of high sensitivity differential scanning calorimetry as a means of predicting drug–excipient interactions. Int J Pharm. 2003;252:235–40.
O’Neill MA, Gaisford S. Application and use of isothermal calorimetry in pharmaceutical development. Int J Pharm. 2011;417:83–93.
Ferraz Pinto M, Afonso de Moura E, Santos de Souza F, Oliveira Macêdo R. Thermal compatibility studies of nitroimidazoles and excipients. J Therm Anal Calorim. 2010;102:323–9.
Oliveira Santos AF, Basilio ID Jr, Souza FS, Medeiros FD, Ferraz Pinto M, de Santana DP, Macêdo RO. Application of thermal analysis of binary mixtures with metformin. J Therm Anal Calorim. 2008;93:361–4.
Chou YP, Huang JY, Tseng JM, Cheng SY, Shu CM. Reaction hazard analysis for the thermal decomposition of cumene hydroperoxide in the presence of sodium hydroxide. J Therm Anal Calorim. 2008;93:275–80.
Sashima ES, Janowska G, Zaborski M, Vnuchkin AV. Compatibility of fibroin/chitosan and fibroin/cellulose blends by thermal analysis. J Therm Anal Calorim. 2007;89:887–91.
Medeiros AFD, Santos AFO, de Souza FS, Jùnior IDB, Valdilânio J, Procópio JVV, de Santana DP, Macêdo RO. Thermal studies of pre-formulates of metronidazole obtained by spray drying technique. J Therm Anal Calorim. 2007;89:775–81.
Silva MAS, Kelmann RG, Foppa T, Cruz AP, Bertol CD, Sartori T, Granada A, Carmignan F, Murakami FS. Thermoanalytical study of fluoxetine hydrochloride. J Therm Anal Calorim. 2007;87:463–7.
Lira AM, Araújo AAS, Basílio IDJ, Santos BLL, Santana DP, Macedo RO. Compatibility studies of lapachol with pharmaceutical excipients for the development of topical formulations. Thermochim Acta. 2007;457:1–6.
Mura P, Furlanetto S, Cirri M, Maestrelli F, Marras AM, Pinzauti S. Optimization of glibenclamide tablet composition through the combined use of differential scanning calorimetry and d-optimal mixture experimental design. J Pharm Biomed Anal. 2005;37:65–71.
Araújo AAS, Storpirtis S, Mercuri LP, Carvalho FMS, dos Santos Filho M, Matos JR. Thermal analysis of the antirretroviral zidovudine (AZT) and evaluation of the compatibility with excipients used in solid dosage forms. Int J Pharm. 2003;260:303–14.
Matos APS, Costa JS, Boniatti J, Seiceira RC, Pitaluga A, Oliveira DL, Vicosa AL, Holandino C. Compatibility study between diazepam and tablet excipients. J Therm Anal Calorim. 2017;127:1675–82.
Liltorp K, Larsen TG, Willumsen B, Holm R. Solid state compatibility studies with tablet excipients using non thermal methods. J Pharm Biomed Anal. 2011;55:424–8.
Monajjemzadeh F, Hassanzadeh D, Valizadeh H, Siahi-Shadbad MR, Mojarrad JS, Robertson TA, Roberts MS. Compatibility studies of acyclovir and lactose in physical mixtures and commercial tablets. Eur J Pharm Biopharm. 2009;73:404–13.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug–excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
da Silva EP, Pereira MAV, de Barros Lima IP, Pereira Barros Lima NG, Guimarães Barbosa E, Soares Aragão CF, Barreto Gomes AP. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim. 2016;123:933–9.
Verma RK, Garg S. Compatibility studies between isosorbide mononitrate and selected excipients used in the development of extended release formulations. J Pharm Biomed Anal. 2004;35:449–58.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Silva LAD, Teixeira FV, Serpa RC, Esteves NL, dos Santos RR, Lima EM, da Cunha-Filho MSS, de Souza Araújo AA, Taveira SF, Marreto RN. Evaluation of carvedilol compatibility with lipid excipients for the development of lipid-based drug delivery systems. J Therm Anal Calorim. 2016;123:2337–44.
Veiga A, Oliveira PR, Bernardi LS, Mendes C, Silva MAS, Sangoi MS, Janissek PR, Murakami FS. Solid-state compatibility studies of a drug without melting point. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6756-8.
Rus LM, Tomuta I, Iuga C, Maier C, Kacso I, Borodi G, Bratu I, Bojita M. Compatibility studies of indapamide/pharmaceutical excipients used in tablet preformulation. Farmacia. 2012;60:92–101.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;35:949–55.
Ding T, Chen L, Zhai LH, Fu Y, Wang-Sun B. Compatibility study of rivaroxaban and its pharmaceutical excipients. J Therm Anal Calorim. 2017;130:1569–73.
Pires SA, Mussel WN, Yoshida MI. Solid-state characterization and pharmaceutical compatibility between citalopram and excipients using thermal and non-thermal techniques. J Therm Anal Calorim. 2017;127:535–42.
Hanko VP, Rohrer JS. Determination of tobramycin and impurities using high-performance anion exchange chromatography with integrated pulsed amperometric detection. J Pharm Biomed Anal. 2006;40:1006–12.
Manyanga V, Elkady E, Hoogmartens J, Adams E. Improved reversed phase liquid chromatographic method with pulsed electrochemical detection for tobramycin in bulk and pharmaceutical formulation. J Pharm Anal. 2013;3:161–7.
Rosasco MA, Segall AI. Determination of the chemical stability of various formulations of tobramycin eye-drops by HPLC method and data analysis by R-GUI stability software. J Appl Pharm Sci. 2015;5:8–13.
The United States Pharmacopeia 38th Ed. (Spanish version) U.S. Pharmacopeial Convention, Rockville; 2015. p. 6020–21.
Tita B, Fulias A, Bandurb G, Marianc E, Tita E. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J Pharm Biomed Anal. 2011;56:221–7.
Zhu B, Padroni M, Colombo G, Phillips G, Crapper J, Young PM, Traini D. The development of a single-use, capsule-free multi-breath tobramycin dry powder inhaler for the treatment of cystic fibrosis. Int J Pharm. 2016;514:392–8.
Benešová K, Pekař M, Lapčík L, Kučerík J. Stability evaluation of n-alkyl hyaluronic acid derivates by DSC and TG measurement. J Therm Anal Calorim. 2006;83:341–8.
Baldino L, Cardea S, Reverchon E. Production of antimicrobial membranes loaded with potassium sorbate using a supercritical phase separation process. Innov Food Sci Emerg. 2016;34:77–85.
de Jager HJ, Prinsloo LC. The dehydration of phosphates monitored DSC/TGA and in situ Raman spectroscopy. Thermochim Acta. 2001;376:187–96.
Dash AK, Suryanarayanan R. Solid-state properties of Tobramycin. Pharmaceut Res. 1991;8:1159–65.
Dash AK. Tobramycin. In: Brittain H, editor. Analytical profiles of drug substances and excipients, vol. 24. San Diego: Academic Press; 1996. p. 579–613.
Munson JW, Hussain A, Bilous R. Precautionary note for use of bisulfite in pharmaceutical formulations. J Pharm Sci. 1977;66:1775–6.
Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press; 2009. p. 654–6.
Akers MJ. Excipient–drug interactions in parenteral formulations. J Pharm Sci. 2002;91:2283–300.
Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press; 2009. p. 242–4.
Martindale, The Complete Drug reference, 32th edn. Pharmaceutical Press, London; 1999. p. 1126.
Kamin W, Schwabe A, Krämer I. Inhalation solutions—which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizers. J Cyst Fibros. 2006;5:2015–213.
Pohanish RP, Greene SA. Wiley guides to chemical incompatibilities. 3rd ed. Hoboken: Wiley; 2009.
Ferrand C, Marc F, Fritsch P, De Saint Blanquat G. Interactions of sorbic acid and sorbates with food amines: role of light, oxygen, temperature and the presence of glycerol and emulsifier. Sci Aliments. 1998;18:603–16.
Acknowledgements
This work was supported by Grant 20020130100342BA from UBA to A. I. Segall.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosasco, M.A., Bonafede, S.L., Faudone, S.N. et al. Compatibility study of tobramycin and pharmaceutical excipients using differential scanning calorimetry, FTIR, DRX, and HPLC. J Therm Anal Calorim 134, 1929–1941 (2018). https://doi.org/10.1007/s10973-018-7282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7282-z