Abstract
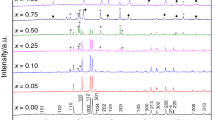
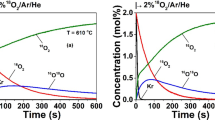
Kinetic analysis of YBaCo4O7+δ, Y0.95Ti0.05BaCo4O7+δ and Y0.15Zr0.1Dy0.75BaCo4O7+δ oxygen carriers for CLAS system has been performed with TG data. The materials are characterized with XRD for phase composition, SEM for particle morphology, and temperature-programmed thermogravimetry for oxidation and reduction reaction analysis. TG experiments are conducted with heating rates of 0.5, 1, and 2 K min−1 in thermal analyzer. The model-fitting approaches, Li Chung-Hsiung and Malek method, are used to estimate the most probable mechanism function. Different processes have different models, for the oxidation process, A1 is selected as the most probable mechanism function for the all samples under different heating rates. And for reduction process, A2/3 is selected as the most probable mechanism function for all samples under different heating rates. That is, nuclei and nuclei growth or their combination are the rate-determining step. In mechanism, activation energy E and pre-exponential factor A are obtained. And the obtained kinetic parameters can reasonably express the oxidation and reduction process.





Similar content being viewed by others
References
Moghtaderi B, Song H. Reduction properties of physically mixed metallic oxide oxygen carriers in chemical looping combustion. Energy Fuels. 2010;24(10):5359–68.
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust Sci. 2005;31(4):283–307.
Ksepko E, Babinski P, Evdou A, Nalbandian L. Studies on the redox reaction kinetics of selected, naturally occurring oxygen carrier. J Therm Anal Calorim. 2016;124(1):1–44.
Wang Y, Wang XY, Hua XN, Zhao CC, Wang W. The reduction mechanism and kinetics of Fe2O3 by hydrogen for chemical-looping hydrogen generation. J Therm Anal Calorim. 2017;129(3):1831–8.
Wang BW, Li HY, Ding D, Shen QW, Zhao HB, Zheng CG. Chemical looping combustion characteristics of coal with Fe2O3 oxygen carrier. J Therm Anal Calorim. 2018;123(1):17–27.
Lyngfelt A, Leckner B, Mattisson T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem Eng Sci. 2001;56(10):3101–13.
Song H, Shah K, Doroodchi E, Wall T, Moghtaderi B. Analysis on chemical reaction kinetics of CuO/SiO2 oxygen carriers for chemical looping air separation. Energy Fuels. 2014;28(1):173–82.
Wang K, Yu QB, Hou LM, Zuo ZL, Qin Q, Ren HL. Simulation and energy consumption analysis of chemical looping air separation system on Aspen Plus. J Therm Anal Calorim. 2016;124(3):1555–60.
Shah K, Moghtaderi B, Wall T. Selection of suitable oxygen carriers for chemical looping air separation: a thermodynamic approach. Energy Fuels. 2012;26(4):2038–45.
Mattisson T, Lyngfelt A, Leion H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int J Greenhouse Gas Control. 2009;3(1):11–9.
Abad A, Adaez-Rubio I, Gaya P, Garc-Labiano F, de Diego LF, Adaez J. Demonstration of chemical-looping with oxygen uncoupling (CLOU) process in a 1.5 kW th continuously operating unit using a Cu-based oxygen carrier. Int J Greenhouse Gas Control. 2012;6(6):189–200.
Arjmand M, Keller M, Leion H, Mattisson T, Lyngfelt A. Oxygen release and oxidation rates of MgAl2O4-supported CuO oxygen carrier for chemical-looping combustion with oxygen uncoupling (CLOU). Energy Fuels. 2012;26(11):6528–39.
Whitty K, Clayton C. Measurement and modeling of kinetics for copper-based chemical looping with oxygen uncoupling. In: Proceedings of the 2nd international conference on chemical looping, Darmstadt, Germany; 26–28 Sept 2012. pp 1–10.
Guo L, Zhao HB, Wang K, Mei DF, Ma ZJ, Zheng CG. Reduction kinetics analysis of sol–gel-derived CuO/CuAl2O4 oxygen carrier for chemical looping with oxygen uncoupling. J Therm Anal Calorim. 2016;123(1):745–56.
Wang K, Yu QB, Qin Q. Reduction kinetics of Cu-based oxygen carriers for chemical-looping air separation. Energy Fuels. 2013;27(9):5466–74.
Hossain MM, de Lasa HI. Reduction and oxidation kinetics of Co–Ni/Al2O3 oxygen carrier involved in a chemical-looping combustion cycles. Chem Eng Sci. 2010;65(1):98–106.
Sedor KE, Hossain MM, de Lasa HI. Reduction kinetics of a fluidizable nickel, alumina oxygen carrier for chemical-looping combustion. Canad J Chem Eng. 2008;86(3):323–34.
Motohashi T, Kadita S, Fjellvag H, Karppinen M, Yamauchi H. Uncommon oxygen intake/release capability of layered cobalt oxides REBaCo4O7+δ: novel oxygen-storage materials. Mater Sci Eng B. 2008;148(1):196–8.
Karppinen M, Yanauchi H, Otani S. Oxygen nonstoichiometry in YBaCo4O7: large low-temperature oxygen absorption/desorption capability. Chem Mater. 2006;18(2):490–4.
Kadita S, Kappinen M, Motohashi T, Yamauchi H. R-site substitution effect on the oxygen-storage capability of RBaCo4O7+δ. Chem Mater. 2008;20(20):6378–81.
Wang S, Hao HS, Zhu BF, Jia JF, Hu X. Modifying the oxygen adsorption properties of YBaCo4O7 by Ca, Al, and Fe doping. J Mater Sci. 2008;43(15):5385–9.
Hao HS, He QL, Cheng YG, Zhao LM. Oxygen adsorption/desorption behavior of YBaCo4O7+δ and its application to oxygen removal from nitrogen. J Rare Earth. 2009;27(5):815–8.
Zhang SM. Study on the oxygen adsorption/desorption properties of doped-YBaCo4O7+δ and its oxygen permeation ability. MA Dissertation, ZhengZhou University, 2011.
Guo LJ. Study on the oxygen adsorption/desorption properties of YBaCo4O7+δ. MA Dissertation, ZhengZhou University, 2005.
Kozeeva LP, Kameneva MY, Lavrov AN, Podberezskaya NV. Synthesis and oxygen behavior of RBaCo4O7+δ (R = Y, Dy-Lu). Inorg Mater. 2013;49(6):626–31.
Parkkima O, Yamauchi H, Karppinen M. Oxygen storage capacity and phase stability of variously substituted YBaCo4O7+δ. Chem Mater. 2013;25(4):599–604.
Valldor M. Syntheses and structures of compounds with YBaCo4O7-type structure. Solid State Sci. 2004;35(28):251–66.
Räsänen S, Motohashi T, Yamauchi H, Kappinen M. Ga-for-Co substitution in YBaCo4O7+δ: effect on high-temperature stability and oxygen-storage capacity. Solid State Ionics. 2012;208:31–5.
Komiyama T, Motohashi T, Masubuchi Y, Kikkawa S. Synthesis, thermal stability, and oxygen intake/release characteristic of YBa(Co1−xAlx)4O7+δ. Mater Res Bull. 2010;45(10):1527–32.
Jung-Hyun K, Arumugam M. Low thermal expansion RBa(Co, M)4O7 cathode materials based on tetrahedral-site cobalt ions for solid oxide fuel cells. Chem Mater. 2010;22(3):822–31.
Rui ZB, Ding JJ, Li F, Lin YS, Li YD. YBaCo4O7+δ sorbent for oxygen-enriched carbon dioxide steam production at a low-temperature. Fuel. 2012;94(1):191–6.
Nagai Y, Yamamoto T, Tanaka T, Yoshida S, Nonaka T, Okamoto T, Suda A, Sugiura M. X-ray absorption fine structure analysis of local structure of CeO2–ZrO2 mixed oxides with the same composition ratio (Ce/Zr = 1). Catal Today. 2002;74(3–4):225–34.
Valldor M, Andersson M. The structure of the new compound YBaCo4O7 with a magnetic feature. Solid State Sci. 2002;4(7):923–31.
Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent CO separations—a review. Chem Eng Sci. 2008;63(18):4433–51.
Jin HG, Okamoto T, Ishida M. Development of a novel chemical-looping combustion: synthesis of a looping material with a double metal oxide of CoO–NiO. Energy Fuels. 1998;12(6):1272–7.
Halikia I, Neou-Syngouna P, Kolitsa D. Isothermal kinetic analysis of the thermal decomposition of magnesium hydroxide using thermogravimetric data. Thermochim Acta. 1998;320(1–2):75–88.
Wang SL, Lin SY, Chen TF. Reaction kinetics of solid-state cyclization of enalapril maleate investigated by isothermal FT-IR microscopic system. Chem Pharm Bull. 2001;49(4):402–6.
Kubaschewsk O, Alcock CB. Metallurgical thermochemistry. 5th ed. Oxford: Pergamon; 1979.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2: part I: low temperature reduction of hematite. Thermochim Acta. 2006;447(1):89–100.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2: part II: low temperature reduction of hematite. Thermochim Acta. 2007;456(2):75–88.
Li CH. An integral approximation formula for kinetic analysis of nonisothermal TGA data. AIChE J. 1985;31(6):1036–8.
Malek J. A computer program for kinetic analysis of non-isothermal thermoanalytical data. Thermochim Acta. 1989;138(2):337–46.
Hossain MM, Quddus MR, de Lasa HI. Reduction kinetics of La modified NiO/La-γAl2O3 oxygen carrier for chemical-looping combustion. Ind Eng Chem Res. 2010;49(21):11009–17.
Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent CO2 separations—a review. AIChE J. 2007;53(7):1817–29.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 51576035, 51604078), the Major State Research Development Program of China (Grant No. 2017YFB0603603), the Fundamental Research Funds for the Central Universities (Grant No. N162504012), and the Post-Doctoral Science Foundation (Grant Nos. 2017M610185, 20170101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, L., Yu, Q., Wang, K. et al. Oxidation and reduction kinetic of YBaCo4O7+δ and substituted oxygen carriers. J Therm Anal Calorim 134, 2213–2221 (2018). https://doi.org/10.1007/s10973-018-7253-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7253-4