Abstract
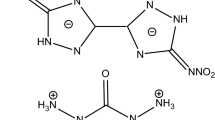
Thermal behaviors of bis(aminofurazano)furazan (BAFF) and bis(nitrofurazano)furazan (BNFF) were studied by the differential scanning calorimetry (DSC) method with a special hermetic high-pressure crucible and compared to that with a common standard Al crucible. The exothermic decomposition processes of the two compounds were completely revealed. The extrapolated onset temperature, peak temperature and enthalpy of exothermic decomposition at the heating rate of 10 °C min−1 are 290.2, 313.4 °C and − 2174 J g−1 for BAFF, and 265.8, 305.0 °C and − 2351 J g−1 for BNFF, respectively. The apparent activation energies of the decomposition process for the two compounds are 115.7 and 131.7 kJ mol−1, respectively. The self-accelerating decomposition temperatures and critical temperatures of thermal explosion are 247.5 and 368.7 °C for BAFF, and 249.6 and 268.1 °C for BAFF, respectively. Both BAFF and BNFF present high thermal stability. The specific heat capacities for the two compounds were determined with the micro-DSC method, and the specific heat capacities and molar heat capacities at 298.15 K are 1.0921 J g−1 K−1 and 257.9 J mol−1 K−1 for BAFF, and 1.0419 J g−1 K−1 and 308.5 J mol−1 K−1 for BNFF, respectively.







Similar content being viewed by others
References
Kinney R, Harwood HJ. The structure of furazan oxides. J Am Chem Soc. 1927;49:514–6.
Olofson RA, Michelman JS. Furazan. J Org Chem. 1965;30:1854–6.
Li ZX, Tang SQ. Review on the synthesis of furoxan derivatives. Chin J Energy Mater. 2006;14(1):77–9.
Huang M, Li HZ, Huang YG, Dong HS. Synthesis of diaminoazofurazan and diaminoazoxyfurazan. Energy Mater. 2004;12:91–4.
Sheremetev AB. Chemistry of furazans fused to five-membered rings. J Heterocycl Chem. 1995;32:371–5.
Sheremetev AB, Kulgina VO, Aleksandrova NS, Dmitriev DE, Strelenko YA, Lebedev VP, Matyushin YN. Dinitro trifurazans with oxy, azo, and azoxy bridges. Propellant Explos Pyrotech. 1998;23:142–8.
Ivanova OA, Averina EB, Kuznetsova TS, Zefirov NS. Synthesis of new 3,4-disubstituted furazans. Chem Heterocycl Commun. 2000;36:1091–6.
Sheremetev AB, Mantseva EV, Dmitriev DE, Sirovskii FS. Transetherification of difurazanyl ethers as a route to unsymmetrical derivatives of difurazanyl ether. Russ Chem Bull. 2002;111:659–62.
Talawar MB, Sivabalan R, Senthilkumar N, Prabhu G, Asthana SN. Synthesis characterization and thermal studies on furazan and tetrazine-based high energy materials. J Hazard Mater. 2004;113:11–5.
Sheremetev AB, Shamshina YL, Dmitriev DE. Synthesis of 3-alkyl-4-aminofurazans. Russ Chem Bull. 2005;54:1032–6.
Sheremetev AB, Palysaeva NV, Struchkova MI. The first synthesis of 3-nitro-4-((s-tetrazin-3-yl)amino)furazans. Mendeleev Commun. 2010;20:350–3.
Chavez D, Klapötke TM, Parrish D, Piercey D, Stierstorfer GJ. The synthesis and energetic properties of 3,4-bis(2,2,2-trinitroethylamino)furazan (BTNEDAF). Propellant Explos Pyrotech. 2014;39:641–8.
Sheremetev AB, Lyalin BV, Kozeev AM, Palysaeva NV, Struchkova MI, Suponitsky KY. A practical anodic oxidation of aminofurazans to azofurazans: an environmentally friendly Route. RSC Adv. 2015;47:37617–9.
Makhova NN, Ovchinnikov IV, Kulikov AS, Khakimov DV, Molchanova MS, Pivina TS. Diaminofuroxan: synthetic approaches and computer-aided study of thermodynamic stability. Propellant Explos Pyrotech. 2012;37:549–57.
Sheremetev AB, Kulagina VO, Ivanova EA. Zero-hydrogen furazan macrocycles with oxy and azo bridges. J Org Chem. 1996;61:1510–2.
Zelenin AK, Trudell ML, Gilardi RD. Synthesis and structure of dinitroazofurazan. J Heterocycl Chem. 1998;35:151–5.
Pagoria PF. A review of energy materials synthesis. Thermochim Acta. 2002;384:187–8.
Sikder AK, Sikder NA. Review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater. 2004;112:1–15.
Zheng W, Wang JN. Review on 3,4-bisnitrofurazanfruoxan (DNTF). Chin J Energy Mater. 2006;14(6):463–4 (in Chinese).
Hu HX, Zhang ZZ, Zhao FQ, Xiao C, Wang QH, Yuan BH. A study on the properties and application of high energy density material 3,4-bisnitrofurazanfruoxan. Acta Amamentarii. 2004;25(2):155–8.
Zhou YS, Zhang ZZ, Li JK, Guan XR, Huang XP, Zhou C. Crystal structure of 3,4-bisnitrofurazanfruoxan. Chin J Explos Propell. 2005;28(2):43–4.
Wang QH. Properties of DNTF-base melting cast explosives. Chin J Explos Propell. 2003;26(3):57–9.
Zhao FQ, Chen P, Hu RZ. Thermochemical properties and non-isothermal decomposition reaction kinetics of 3,4-bisnitrofurazanfruoxan. J Hazard Mater. 2004;113:67–71.
Zhao FQ, Chen P, Luo Y. Study on the composite modified double base propellant containing 3,4-bisnitrofurazanfruoxan. J Propuls Technol. 2004;26(6):570–3.
Wang J, Dong HS, Huang YG, Li JS. Studies on the preparation and crystal structure of 3,4-diaminofurazanfruoxan. Acta Chim Sin. 2006;64(2):158–62.
Zhang Y, Zhou C, Wang BZ, Zhou YS, Xu KZ, Jia SY, Zhao FQ. Synthesis and characteristics of bis(nitrofurazano)furazan (BNFF), an insensitive material with high energy-density. Propellant Explos Pyrotech. 2014;39:809–14.
Wang XJ, Xu KZ, Sun Q, Wang BZ, Zhou C, Zhao FQ. The insensitive energetic material trifurazano-oxacycloheptatriene (TFO): synthesis and detonation properties. Propellant Explos Pyrotech. 2014;40:9–12.
Kim TK, Choe JH, Lee BW, Chung KH. Synthesis and characterization of BNFF analogues. Bull Korean Chem Soc. 2012;33:2765–8.
Zhao K, Wang HX, Jiang QL, Liu RP. The research on volatility of 3,4-bisnitrofurazanfruoxan. Sci Tech Eng. 2014;14(29):271–3.
Mukherjee I, Rosolen M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J Therm Anal Calorim. 2013;114:1161–6.
Zhong Y, Li X, Gu Z, Wang X, Yang L, Yang X, Zhang Z, Zhong B. Thermal studies on Li(CH3CN)4PF6 and Li(C4H10O2)2PF6 complexes by the TG–DTA–MS and DSC. J Therm Anal Calorim. 2018;131:1287–93.
Muñoz-Sánchez B, Nieto-Maestre J, Imbuluzqueta G, MarañónIñigo I, Iparraguirre-Torres I, García-Romero A. A precise method to measure the specific heat of solar salt-based nanofluids. J Therm Anal Calorim. 2017;129:905–10.
Zhang Y, Wu H, Xu KZ, Zhang WT, Song JR, Zhao FQ, Hu RZ. Thermolysis, non-isothermal decomposition kinetics, specific heat capacity and adiabatic time-to-explosion of (Cu(NH3)4)(DNANT)2 [DNANT = Dinitroacetonitrile). J Phys Chem A. 2014;118:1168–74.
Ditmars DA, Ishihara S, Chang SS, Bernstein G, West ED. Enthalpy and heat-capacity standard reference material: synthetic sapphire (alpha-Al-2O3) from 10 to 2250 K. J Res Natl Bur Stand. 1982;87(2):159–63.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–5.
Ozawa TA. Method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Zhang TL, Hu RZ, Xie Y, Li FP. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC. Thermochim Acta. 1994;244:171–6.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008 (in Chinese).
Xu KZ, Song JR, Zhao FQ, Ma HX, Gao HX, Chang CR, Ren YH, Hu RZ. Thermal behavior, specific heat capacity and adiabatic time-to-explosion of G(FOX-7). J Hazard Mater. 2008;158:333–7.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21673178).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, ZC., Du, MY., Zhai, LJ. et al. Hermetic thermal behaviors and specific heat capacities of bis(aminofurazano)furazan and bis(nitrofurazano)furazan. J Therm Anal Calorim 133, 1379–1385 (2018). https://doi.org/10.1007/s10973-018-7242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7242-7