Abstract
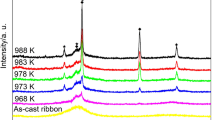
In this work, the isothermal crystallization kinetics of cost-effective Fe75Cr5P9B4C7 metallic glass with a combination of desired merits synthesized by industrial ferro-alloys without high-purity materials was evaluated by Johnson–Mehl–Avrami approach using differential scanning calorimeter. The Avrami exponents at all isothermal annealing temperatures range from about 2.93 to 4.61, indicating a three-dimensional diffusion-controlled growth with an increasing nucleation rate during the isothermal crystallization. Meanwhile, the Avrami exponent firstly increases from 2.93 at the initial time to a maximum value of 4.61 and then decreases to 4.09 with the increment of the isothermal annealing temperature, which can be attributed to the atomic diffusion in the alloy. Additionally, the trend of the local Avrami exponent variations at different isothermal annealing temperatures reflects a variable crystallization mechanism during the crystallization process. Moreover, the local activation energy determined by Arrhenius equation gradually decreases from about 412 to 383 kJ mol−1 during the present isothermal crystallization, further revealing that the process is dominated by a three-dimensional diffusion-controlled growth with an increasing nucleation rate, which provides useful insights into the formation of the present alloy.







Similar content being viewed by others
References
Suryanarayana C, Inoue A. Iron-based bulk metallic glasses. Int Mater Rev. 2013;58:131–66.
Pang SJ, Zhang T, Asami K, Inoue A. Bulk glassy Fe–Cr–Mo–C–B alloys with high corrosion resistance. Corros Sci. 2002;44:1847–56.
Shen J, Chen QJ, Sun JF, Fan HB, Wang G. Exceptionally high glass-forming ability of an FeCoCrMoCBY alloy. Appl Phys Lett. 2005;86:151907.
Shen BL, Inoue A, Chang CT. Super high strength and good soft-magnetic properties of (Fe, Co)–B–Si–Nb bulk glassy alloys with high glass-forming ability. Appl Phys Lett. 2004;85:4911–3.
Ponnambalam V, Poon SJ, Shiflet GJ, Keppens VM, Taylor R, Petculescu G. Synthesis of iron-based bulk metallic glasses as nonferromagnetic amorphous steel alloys. Appl Phys Lett. 2003;83:1131–3.
Zhang T, Liu FJ, Pang SJ, Li R. Ductile Fe-based bulk metallic glass with good soft-magnetic properties. Mater Trans. 2007;48:1157–60.
Wang JF, Li R, Hua NB, Huang L, Zhang T. Ternary Fe–P–C bulk metallic glass with good soft-magnetic and mechanical properties. Scr Mater. 2011;65:536–9.
Shi MJ, Pang SJ, Zhang T. Towards improved integrated properties in FeCrPCB bulk metallic glasses by Cr addition. Intermetallics. 2015;61:16–20.
Inoue A, Shinohara Y, Gook JS. Thermal and magnetic properties of bulk Fe-based glassy alloys prepared by copper mold casting. Mater Trans JIM. 1995;36:1427–33.
Shen TD, Schwarz RB. Bulk ferromagnetic glasses prepared by flux melting and water quenching. Appl Phys Lett. 1999;75:49–51.
Chang CT, Shen BL, Inoue A. Synthesis of bulk glassy alloys in the (Fe Co, Ni)–B–Si–Nb system. Mater Sci Eng A. 2007;449–451:239–42.
Jung HY, Yi S. Enhanced glass forming ability and soft magnetic properties through an optimum Nb addition to a Fe–C–Si–B–P bulk metallic glass. Intermetallics. 2010;18:1936–40.
Li HX, Kim KB, Yi S. Enhanced glass-forming ability of Fe-based bulk metallic glasses prepared using hot metal and commercial raw materials through the optimization of Mo content. Scr Mater. 2007;56:1035–8.
Yang YJ, Xing DW, Shen J, Sun JF, Wei SD, He HJ, McCartney DG. Crystallization kinetics of a bulk amorphous Cu–Ti–Zr–Ni alloy investigated by differential scanning calorimetry. J Alloys Compd. 2006;415:106–10.
Qiao JC, Pelletier JM. Crystallization kinetics in Cu46Zr45Al7Y2 bulk metallic glass by differential scanning calorimetry (DSC). J Non Cryst Solids. 2011;357:2590–4.
Liu L, Wu ZF, Zhang J. Crystallization kinetics of Zr55Cu30Al10Ni5 bulk amorphous alloy. J Alloys Compd. 2002;339(1–2):90–5.
Sun H, Jian ZY, Jiang BQ, Gao Q. Study on glass transition temperature and kinetics of Cu–Zr glassy alloys. J Therm Anal Calorim. 2017;129:1429–33.
Prajapati SR, Kasyap S, Pate AT, Pratap A. Non-isothermal crystallization kinetics of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim. 2016;124:21–33.
Gong P, Yao KF, Zhao SF. Cu-alloying effect on crystallization kinetics of Ti41Zr25Be28Fe6 bulk metallic glass. J Therm Anal Calorim. 2015;121:697–704.
Taghvaei AH, Stoica M, Song KK, Janghorban K, Eckert J. Crystallization kinetics of Co40Fe22Ta8B30 glassy alloy with high thermal stability and soft magnetic properties. J Alloys Compd. 2014;605:199–207.
Taghvaei AH, Eckert J. A comparative study on the isochronal and isothermal crystallization kinetics of Co46.45Fe25.55Ta8B20 soft magnetic metallic glass with high thermal stability. J Alloys Compd. 2016;675:223–30.
Illeková E. The crystallization kinetics of Fe80Si4B16metallic glass. Thermochim Acta. 1996;280–281:289–301.
Bayri N, Izgi T, Gencer H, Sovak P, Gunes M, Atalay S. Crystallization kinetics of Fe73.5−xMnxCu1Nb3Si13.5B9 (x = 0, 1, 3, 5, 7) amorphous alloys. J Non Cryst Solids. 2009;355:12–6.
Biswas K, Venkataraman S, Zhang WY, Ram S, Eckert J. Glass-forming ability and fragility parameter of amorphous Fe67Co9.5Nd3Dy0.5B20. J Appl Phys. 2006;100:023501.
Zhang JT, Wang WM, Ma HJ, Li GH, Li R, Zhang ZH. Isochronal and isothermal crystallization kinetics of amorphous Fe-based alloys. Thermochim Acta. 2010;505:41–6.
Mitrovic N, Roth S, Eckert J. Kinetics of the glass-transition and crystallization process of Fe72-xNbxAl5Ga2P11C6B4 (x = 0, 2) metallic glasses. Appl Phys Lett. 2001;78:2145–7.
Jung HY, Stoica M, Yi S, Kim DH, Eckert J. Crystallization kinetics of Fe76.5−xC6.0Si3.3B5.5P8.7Cux (x = 0, 0.5, and 1 at. pct) bulk amorphous alloy. Mater Trans A. 2015;46A:2415–21.
Jung HY, Yi S. Effect of Cu addition on nanocrystallization behaviors and magnetic properties of the Fe76.5−xC6.0Si3.3B5.5P8.7Cux (x = 0–3 at.%) bulk metallic glass. J Alloys Compd. 2013;561:76–81.
Stoica M, Kumar S, Roth S, Ram S, Eckert J, Vaughan G, Yavari AR. Crystallization kinetics and magnetic properties of Fe66Nb4B30 bulk metallic glass. J Alloys Compd. 2009;483:632–7.
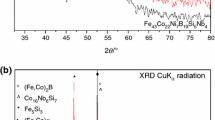
Xu T, Jian ZY, Chang FE, Zhuo LC, Shi MJ, Zhu M, Xu JF, Liu YQ, Zhang T. Synthesis of Fe75Cr5(PBC)20 bulk metallic glasses with a combination of desired merits using industrial ferro-alloys without high-purity materials. J Alloys Compd. 2017;699:92–7.
Wang JF, Di YX, Fang Z, Guan SK, Zhang T. Thermal stability, crystallization and soft magnetic properties of Fe–P–C-based glassy alloys. J Non Cryst Solids. 2016;454:39–45.
Han JJ, Wang CP, Kou SZ, Liu XJ. Thermal stability, crystallization behavior, Vickers hardness and magnetic properties of Fe–Co–Ni–Cr–Mo–C–B–Y bulk metallic glasses. Trans Nonferrous Met Soc China. 2013;23:148–55.
Chen LC, Spaepen F. Calorimetric evidence for the micro-quasicrystalline structure of ‘amorphous’ Al/transition metal alloys. Nature. 1988;336:366–8.
Johnson WA, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans Am Inst Min Metall Eng. 1939;135:416–58.
Avrami M. Kinetics of phase change. I general theory. J Chem Phys. 1939;7:1103–12.
Ranganathan S, Heimendahi MV. The three activation energies with isothermal transformations: applications to metallic glasses. J Mater Sci. 1981;16(9):2401–4.
Révész Á. Crystallization kinetics and thermal stability of an amorphous Fe77C5B4Al2GaP9Si2 bulk metallic glass. J Therm Anal Calorim. 2008;91:879–84.
Sun YD, Li ZQ, Liu JS, Yang JN, Cong MQ. Crystallization kinetics of Mg61Cu28Gd11 and (Mg61Cu28Gd11)99.5Sb0.5 bulk metallic glasses. J Alloys Compd. 2010;506:302–7.
Calka A, Radinski AP. Decoupled bulk and surface crystallization of Pd85Si15 glassy metallic alloys: description of isothermal crystallization by a local value of the Avrami exponent. J Mater Res. 1988;3(1):59–66.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant Nos. 51671151, 51371133, 51401156 and 51604223) and the Science and Technology Program of Shaanxi Province (Grant No. 2016KJXX-87).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, T., Jian, Z., Chang, F. et al. Isothermal crystallization kinetics of Fe75Cr5P9B4C7 metallic glass with cost-effectiveness and desirable merits. J Therm Anal Calorim 133, 1309–1315 (2018). https://doi.org/10.1007/s10973-018-7208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7208-9