Abstract
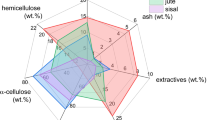
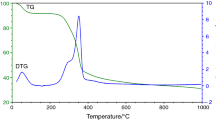
Raw and pyrolyzed samples of the plane tree seeds (PTS) were tested by various advanced analytical techniques including simultaneous TG-DSC technique, FTIR analysis, X-ray diffraction (XRD) analysis, Raman spectroscopy analysis, GC–MS (gas chromatography–mass spectrometry) analysis and scanning electron microscope analysis, for its characterization procedure and the pre-treatments in possible application in CCS. Nondestructive analytical method (XRD) showed that raw material is typical for carbon-rich material, where was identified increase in interlayer spacing within graphite structure. The XRD results of pyrolyzed sample at 850 °C showed a sudden loss in interlayer spacing. Spectroscopic analyses of pyrolyzed sample demonstrated the presence of typical aromatic structures found in amorphous carbon. Results indicate the high levels of the growth in basal planes of graphite structure in pyrolyzed sample. It was established that integrated reaction model parameters for pyrolysis of untreated PTS sample realistically describe active temperature period required for charcoal forming, under non-isothermal conditions. It was found that mechanical treatment of material results in increase in the number of chemical compounds. Micrograph showed the presence of variety of shapes and structures, where after pyrolysis, some dissipated pores were detected. One of these pores was partially blocked in some places, depending on the size of surface area. The results showed that the resulting char has very good features for further activation process, while the PTS would represent a good candidate in its application in the CCS.






Similar content being viewed by others
References
Carapellucci R, Giordano L, Vaccarelli M. The use of biomass to reduce power derating in combined cycle power plants retrofitted with post-combustion CO2 capture. Energy Convers Manag. 2016;107:52–9.
Qadir A, Sharma M, Parvareh R, Khalilpour R, Abbas A. Flexible dynamic operation of solar-integrated power plant with solvent based post-combustion carbon capture (PCC) process. Energy Convers Manag. 2015;97:7–19.
Oboirien BO, Thulari V, North BC. Enrichment of trace elements in bottom ash from coal oxy-combustion: effect of coal types. Appl Energy. 2016;177:81–6.
Yu C-H, Huang C-H, Tan C-S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual Res. 2012;12:745–69.
Scholes CA, Anderson CJ, Stevens GW, Kentish SE. Membrane gas separation—physical solvent absorption combined plant simulations for pre-combustion capture. Energy Proc. 2013;37:1039–49.
Mustafa J, Farhan M, Hussain M. CO2 separation from flue gases using different types of membranes. J Membr Sci Technol. 2016;6:153–9.
Herzog HJ. The economics of CO2 separation and capture. Cambridge: MIT Energy Laboratory Cambridge; 2000. p. 3–19.
Chatterjee S, Rayalu S, Kolev SD, Krupadam RJ. Adsorption of carbon dioxide on naturally occurring solid amino acids. J Environ Chem Eng. 2016;4:3170–6.
Wang J, Huang L, Yang R, Zhang Z, Wu J, Gao Y, Wang Q, O’Hare D, Zhong Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ Sci. 2014;7:3478–518.
Hart A, Gnanendran N. Cryogenic CO2 capture in natural gas. Energy Proc. 2009;1:697–706.
Zulfiqar S, Sarwar MI, Mecerreyes D. Polymeric ionic liquids for CO2 capture and separation: potential, progress and challenges. Polym Chem. 2015;6:6435–51.
Park Y, Andrew Lin K-Y, Allisa Park A-H, Petit C. Recent advances in anhydrous solvents for CO2 capture: ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front Energy Res. 2015;3:1–14.
Rydén M, Ramos P. H2 production with CO2 capture by sorption enhanced chemical-looping reforming using NiO as oxygen carrier and CaO as CO2 sorbent. Fuel Process Technol. 2012;96:27–36.
Duan W, Yu Q. Thermodynamic analysis of hydrogen-enriched syngas generation coupled with in situ CO2 capture using chemical looping gasification method. J Therm Anal Calorim. 2018;131(2):1671–80.
Adoption of the Paris agreement—by the President—Draft decision -/CP.21, UNFCCC. 12 December 2015, Archived from the original on 12 December 2015, Retrieved 2015-12-12, Available in Pdf document.
Parshetti GK, Chowdhury S, Balasubramanian R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel. 2015;148:246–54.
Jiménez V, Ramírez-Lucas A, Antonio Díaz J, Sánchez P, Romero A. CO2 capture in different carbon materials. Environ Sci Technol. 2012;46:7407–14.
Przepiórski J, Czyżewski A, Pietrzak R, Tryba B. MgO/CaO-loaded porous carbons for carbon dioxide capture: effects accompanying regeneration process. J Therm Anal Calorim. 2013;111(1):357–64.
Correia LB, Fiuza RA Jr, de Andrade RC, Andrade HMC. CO2 capture on activated carbons derived from mango fruit (Mangifera indica L) seed shells: a TG study. J Therm Anal Calorim. 2018;131(1):579–86.
Vargas DP, Giraldo L, Erto A, Moreno-Piraján JC. Chemical modification of activated carbon monoliths for CO2 adsorption. J Therm Anal Calorim. 2013;114(3):1039–47.
Zhong L, Zhang Y, Ji Y, Norris P, Pan W-P. Synthesis of activated carbon from coal pitch for mercury removal in coal-fired power plants. J Therm Anal Calorim. 2016;123(1):851–60.
Gül A, Topay M, Özaltın O. The important of urban forests toward to global warming threat. In: International Davraz congress on social and economic issues shaping the world’s future: new global dialogue, ISBN 978 9944 452 33 5, 24–27 September 2009, Isparta, 2009, pp. 221-234.
Rumble H, Rogers K, Doick K, Hutchings T. Valuing Wrexham’s urban forests. Assessing the ecosystem services of Wrexham’s urban trees: a technical report. The Research Agency of the Forestry Commission, North Wales, Wales, June 2014, 2014, pp. 21–31.
Plaza MG, Pevida C, Arias B, Fermoso J, Arenillas A, Rubiera F, Pis JJ. Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J Therm Anal Calorim. 2008;92(2):601–6.
Bukalak D, Majchrzak-Kucęba I, Nowak W. Assessment of the sorption capacity and regeneration of carbon dioxide sorbents using thermogravimetric methods. J Therm Anal Calorim. 2013;113(1):157–60.
ASTM D5373-14e2. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke, ASTM International, West Conshohocken, PA, USA, 2014.
ISO 1928. Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value, USA (standard has been revised by: ISO 1928:2009), 1995.
TAPPI/ANSI T 257 cm-12. Sampling and preparing wood for analysis, Test Method, USA, 2013.
T 264 cm-97. Preparation of wood for chemical analysis, Test Method, USA, 2013.
Browning BL. Methods of wood chemistry, vol. 2. New York: Interscience Publishers; 1967. p. 561–87.
Schoening AG, Johansson G. Absorptiometric determination of acid-soluble lignin in semichemical bisulfite pulps and in some woods and plants. Svensk Papperstid. 1965;68:607–13.
TAPPI UM 250. Acid-soluble Lignin in Wood and Pulp, TAPPI Useful Methods, Tappi, Atlanta, GA, USA, 1991.
TAPPI T 207 cm-99. Water Solubility of Wood and Pulp, TAPPI Useful Methods, Tappi, Atlanta, GA, USA, 1999.
ASTM D1102-84. Standard Test Method for Ash in Wood, ASTM International, West Conshohocken, PA, USA, 2013.
Narayan R, Antal MJ. Thermal lag, fusion, and the compensation effect during biomass pyrolysis. Ind Eng Chem Res. 1996;35(5):1711–21.
Tarasov A. Thermal analysis: methods, principles, application. lecture on thermal analysis. Lecture series heterogeneous catalysis, FHI MPG, AC FHI Publication, 2012, pp. 1–48.
Meng H, Wang S, Chen L, Wu Z, Zhao J. Thermal behavior and the evolution of char structure during co-pyrolysis of platanus wood blends with different rank coals from northern China. Fuel. 2015;158:602–11.
El May Y, Jeguirim M, Dorge S, Trouvé G, Said R. Thermogravimetric analysis and kinetic study on palm of phoenix dactylifera L. In: The 7th Mediterranean Combustion Symposium, Chia Laguna, Cagliari, Sardinia, Italy, Sept 11–15, 2011, pp. 1–9.
Bendahou A, Dfresne A, Kaddami H, Habibi Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohyd Polym. 2007;68:601–8.
Strezov V. System approach to biomass pyrolysis: product characterization. In: Din P, Lorenz P, editors. Bionature 2012: The 3rd international conference on bioenvironment, biodiversity and renewable energies, iaria conference. St. Maarten, Netherlands, Antilles, March 25-30, 2012, pp. 7–11.
Gu L, Wu S, Li B, Wen H, Zhang D, Ye J, Wang L. Persulfate oxidation assisted hydrochar production from Platanus Orientalis Leaves: physicochemical and combustion characteristics. Biores Technol. 2017;244:517–24.
Vhathvarothai N, Ness J, Yu QJ. An investigation of thermal behaviour of biomass and coal during copyrolysis using thermogravimetric analysis. Int J Energy Res. 2014;38:1145–54.
Rowell RM, Pettersen R, Tshabalala MA. Cell wall chemistry. In: Rowell RM, editor. Handbook of wood chemistry and wood composites. 2nd ed. Boca Raton: CRC Press; 2013. p. 33–75.
Emandi A, Vasiliu CI, Budrugeac P, Stamatin I. Quantitative investigation of wood composition by integrated FT-IR and thermogravimetric methods. Cellulose Chem Tech. 2011;45:579–84.
Ejikeme EM, Ejikeme PCN, Abalu BN. Equilibrium, kinetics and thermodynamics studies on MB adsorption using hamburger seed shell activated carbon. Int J Eng Tech. 2014;14:74–83.
Assis MR, Brancheriau L, Napoli A, Trugilho PF. Factors affecting the mechanics of carbonized wood: literature review. Wood Sci Technol. 2016;50:519–36.
Viswanathan B, Neel PI, Varadarajan TK. Development of carbon materials for energy and environmental applications. Catal Surv Asia. 2009;13:164–83.
Hassan MM, Schiermeister L, Staiger MP. Thermal, chemical and morphological properties of carbon fibres derived from chemically pre-treated wool fibres. RSC Adv. 2015;5:55353–62.
Lehman JH, Terrones M, Mansfield E, Hurst KE, Meunier V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon. 2011;49:2581–602.
Antal MJ, Grønli M. The art, science, and technology of charcoal production. Ind Eng Chem Res. 2003;42:1619–40.
Almeida G, Brito JO, Perre P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: the potential of mass loss as a synthetic indicator. Biores Technol. 2010;101:9778–84.
Bergman P, Boersma A, Zwart R, Kiel J. Torrefaction for biomass co-firing in existing coal-fired power stations. Energy Research Centre of the Netherlands, Report No. ECN-C-05-013, 2005.
Oyedun AO, Lam KL, Hui CW. Charcoal production via multistage pyrolysis. Chin J Chem Eng. 2012;20:455–60.
Chu PK, Li L. Characterization of amorphous and nanocrystalline carbon films. Mater Chem Phys. 2006;96:253–77.
Zhang S, Min ZH, Tay HL, Asadullah M, Li CZ. Effects of volatile-char interactions on the evolution of char structure during the gasification of Victorian brown coal in steam. Fuel. 2011;90:1529–35.
Guo X, Tay HL, Zhang S, Li CZ. Changes in char structure during the gasification of a Victorian brown coal in steam and oxygen at 800 & #xB0;C. Energy Fuels. 2008;22:4034–8.
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri M, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK. Raman spectrum of graphene and graphene layers. Phys Rev Lett. 2006;97:187401.
Tuinstra F, Koenig JL. Raman spectrum of graphite. J Chem Phys. 1970;53:1126–30.
Lähdetie A. Wood biomass characterization by Raman spectroscopy, school of chemical technology, Department of Forest Products Technology, Aalto University, Doctoral Dissertations, Aalto University publication series, ISBN 978-952-60-5468-1, Helsinki, Finland, 2013, pp. 1–67.
Pakdel H, Roy C. Production and characterization of carboxylic acids from wood. Part I: low molecular weight carboxylic acids. Biomass. 1987;13:155–71.
Pakdel H, Zhang H-G, Roy C. Production and characterization of carboxylic acids from wood. Part II: High molecular weight fatty acid and resin acids. Biores Technol. 1994;47:45–53.
Yan QL, Gozin M, Zhao FQ, Cohen A, Pang SP. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale. 2016;8:4799–851.
Alvarez J, Amutio M, Lopez G, Nunes J, Olazar M, Bilbao J. Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon. Biores Technol. 2014;170:132–7.
Dodevski V, Stojmenović M, Vujković M, Krstić J, Krstić S, Bajuk-Bogdanović D, Kuzmanović B, Kaluđerović B, Mentus S. Complex insight into the charge storage behavior of active carbons obtained by carbonization of the plane tree seed. Electrochim Acta. 2016;222:156–71.
Fu P, Hu S, Xiang J, Sun L, Su S, Wang J. Evoluation of the porous structure development of chars from pyrolysis of rice straw: effects of pyrolysis temperature and heating rate. Fuel. 2012;98:177–83.
Angin D, Şensöz S. Effect of pyrolysis temperature on chemical and surface properties of biochar of rapeseed (Brassica napus L). Int J Phytoremed. 2014;16:684–93.
Moralı U, Şensöz S. Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: characterization of bio-oil and bio-char. Fuel. 2015;150:672–8.
Özçimen D, Ersoy-Meriçboyu A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew Energy. 2010;35:1319–24.
Acknowledgements
This research work was partially supported by the Serbian Ministry of Education, Science and Technological Development under the projects numbered as 172015 and III45005. The authors would also like to thank Dr. Smilja Marković for taking thermo-analytical measurements on a commercial device.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janković, B., Dodevski, V., Stojmenović, M. et al. Characterization analysis of raw and pyrolyzed plane tree seed (Platanus orientalis L.) samples for its application in carbon capture and storage (CCS) technology. J Therm Anal Calorim 133, 465–480 (2018). https://doi.org/10.1007/s10973-018-7207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7207-x